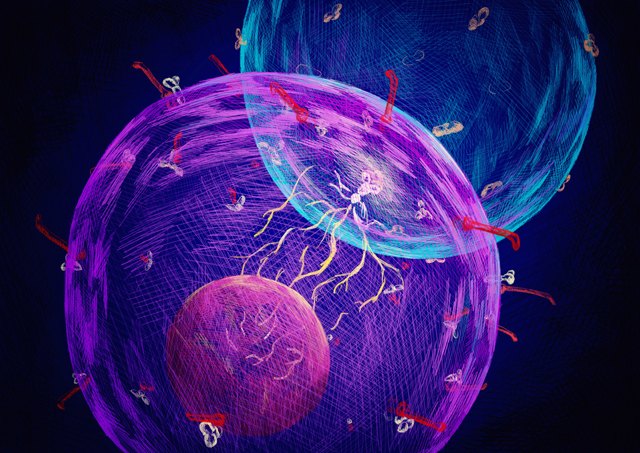
 Conceptual illustration of a designer cell sensing a target cellRYO TACHIBANA, GRADUATE SCHOOL OF PHARMACEUTICAL SCIENCES, THE UNIVERSITY OF TOKYOEngineering an immune cell to recognize and kill a cancer cell is the key to chimeric antigen receptor (CAR) T-cell therapy, but modified immune cells also have the potential to cause problems for patients. One such complication, cytokine release syndrome, is an overreaction of the immune system that can cause symptoms as mild as a fever and as serious as organ dysfunction and death. In a study published today (November 13) in Nature Chemical Biology, researchers have generated nonimmune cells with the ability to kill cancer cells on contact.
Conceptual illustration of a designer cell sensing a target cellRYO TACHIBANA, GRADUATE SCHOOL OF PHARMACEUTICAL SCIENCES, THE UNIVERSITY OF TOKYOEngineering an immune cell to recognize and kill a cancer cell is the key to chimeric antigen receptor (CAR) T-cell therapy, but modified immune cells also have the potential to cause problems for patients. One such complication, cytokine release syndrome, is an overreaction of the immune system that can cause symptoms as mild as a fever and as serious as organ dysfunction and death. In a study published today (November 13) in Nature Chemical Biology, researchers have generated nonimmune cells with the ability to kill cancer cells on contact.
“We’re entering a new era of cell-based therapies, and this is the first to extend the scope beyond lymphocytes per se,” Simon Davis, a biologist at the University of Oxford in the U.K. who was not involved in the study, writes in an email to The Scientist. “The authors show that they can turn a passive, nonimmune cell into a contact-dependent killer of breast cancer cells,” he adds.
I liken this to an explosion, which could eventually kill the synthetic T cell, along with a couple of other cells around, mostly cancer cells.—Martin Fussenegger,
ETH Zurich
Typical immunotherapies work by harnessing the power of the immune system. In CAR T-cell therapy, for example, patients receive a transfusion of their own T cells that have been ...




















