Scientists use retrosynthesis to deconstruct a desired target compound into its building blocks and plan the optimal route for therapeutic synthesis.
©iStock, marcoventuriniautieri
In early stage drug manufacturing research, scientists develop synthetic routes to build active pharmaceutical ingredients (APIs). This can be a painstakingly complex process, with traditional route design methods that rely heavily on human expertise, labor-intensive experiments, and trial-and-error approaches producing suboptimal and costly chemical reaction pathways.1 Computer-assisted retrosynthesis technologies that are supported by artificial intelligence (AI) are revolutionizing route design, enabling safer, more sustainable, and green-by-design APIs.2
What Is Retrosynthesis?
Retrosynthesis or retrosynthetic analysis (RA) is a strategic organic chemistry approach that scientists use to construct target molecules from easy-to-make or readily available precursors.2 Following chemical reaction rules to deconstruct a desired product into reactants, this method helps chemists plan synthesis pathways for natural products, APIs, and other complex molecules.1
Traditionally, deconstruction rules have been dictated by human experts, requiring specialized knowledge of synthetic methodologies. For example, human experts need to know how to break chemical structures into the necessary fragments and add the correct functional groups for a reaction to take place. Synthetic chemists may reiterate this process until a structure cannot be further simplified or until they determine a desired route. This dependence on manual reiteration and expertise results in significant resource waste with respect to time, cost, and materials, which has been a barrier to improving route design.1
A Need for Greener Chemistry
Greener chemistry is critical to developing safe and effective APIs as well as downstream therapeutics. Scientists seek greener-by-design synthetic routes that inherently reduce reaction steps, maximize yield, identify greener pathways, and minimize side products and impurities. Combined with green-by-design strategies, computer-aided retrosynthesis can help researchers address route planning challenges, reducing the need for traditional intensive experimentation and lowering development and production costs
Computer-Aided Retrosynthesis
Computational, data-driven processes increasingly help scientists determine the most efficient retrosynthetic routes. Instead of experimentally determining synthetic pathways to create APIs, researchers use computer-aided retrosynthesis to virtually construct targets from reactant databases.1 Computational tools that integrate AI and machine learning-based techniques can automate key planning and reiteration steps, further accelerating chemistry research and development, including retrosynthesis.3
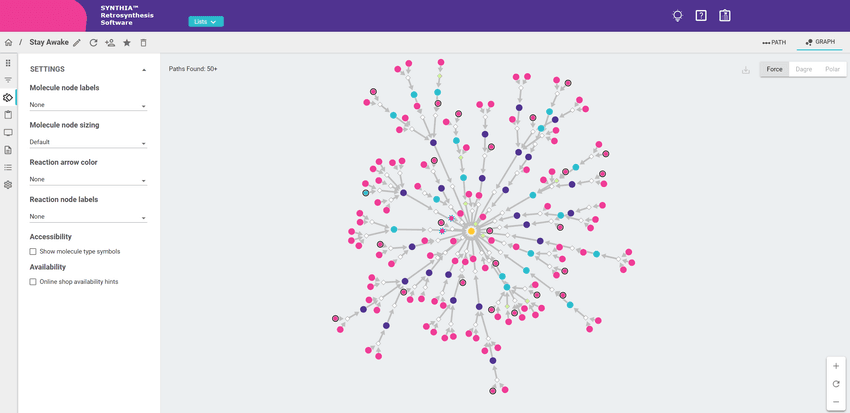
Researchers can use computer-aided retrosynthesis to explore unique and innovative API-building routes.
Merck KGaA, Darmstadt, Germany
SYNTHIA™ from Merck KGaA, Darmstadt, Germany is a software platform that employs a hybrid computational retrosynthesis approach, integrating chemist-encoded rules with machine learning algorithms engineered by computer scientists. With a catalog of over 12 million commercially available starting materials and building blocks, SYNTHIA™ allows scientists to quickly discover novel pathways for new and published target molecules. It offers an unbiased, objective, computer-aided approach to simplifying robust synthesis pathway discovery. Researchers can quickly and efficiently scan hundreds of pathways to better identify the best option according to their specific needs.
SYNTHIA™ also provides pathways that utilize enzymes to achieve more sustainable chemical transformations. Its database offers information on reaction conditions, enzyme details, and reference links. Importantly, this supports biocatalysis, a key component of green chemistry. Additionally, SYNTHIA™ incorporates building block sustainability information with clear in-pathway labels, helping researchers design greener synthesis routes.
From experienced synthetic chemists to budding drug design researchers, pairing computer-aided retrosynthesis and green chemistry helps scientists streamline route planning, expediting API development and reducing financial and environmental manufacturing costs.
- Watson IA, et al. A retrosynthetic analysis algorithm implementation. J Cheminform. 2019;11(1):1.
- Teixeira RI, et al. Computer-aided retrosynthesis for greener and optimal total synthesis of a helicase-primase inhibitor active pharmaceutical ingredient. JACS Au. 2024;4(11):4263-4272.
- Back S, et al. Accelerated chemical science with AI. Digit Discov. 2023;3(1):23-33.
















