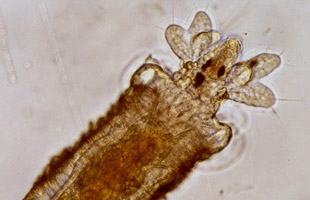
 Juvenile Hydroides elegans tubeworm, post-metamorphosisCOURTESY OF BRIAN NEDVEDHydroides elegans is a tiny marine tubeworm that causes millions of dollars in increased fuel costs each year by settling on the hulls of ships and creating drag. Before the organism settles on a surface, though, it must receive an as yet unknown signal to transition from the free-swimming larval stage that precedes settling of the juvenile tubeworm.
Juvenile Hydroides elegans tubeworm, post-metamorphosisCOURTESY OF BRIAN NEDVEDHydroides elegans is a tiny marine tubeworm that causes millions of dollars in increased fuel costs each year by settling on the hulls of ships and creating drag. Before the organism settles on a surface, though, it must receive an as yet unknown signal to transition from the free-swimming larval stage that precedes settling of the juvenile tubeworm.
Now, researchers have demonstrated that a set of bacterial genes necessary for H. elegans metamorphosis encodes components of structures that resemble the contractile tails of bacterial viruses or phage. These bacterial metamorphosis-associated contractile structures, or MACs, form an organized extracellular array that is required for the switch from free-swimming larva to anchored juvenile tubeworm. The work was published today (January 9) in Science.
“I have no question that this is a benchmark paper in biology,” said Margaret McFall-Ngai, a professor of medical microbiology and immunology at the University of Wisconsin-Madison, who was not involved in the work. “For many, many decades people have been . . . trying to figure out how and why marine larvae settle where they do in the environment.”
Michael Hadfield, ...





















