Stem cells can self-renew and differentiate into a wide variety of cell types, making them critical components in embryonic development. In adults, stem cell populations, such as those in the intestines, could turn into tumor cells and lead to the development of colorectal cancer. Ongoing research efforts have allowed scientists to better understand this transformation process, getting them closer to the possibility of treating this aggressive and often fatal type of cancer. Other breakthroughs in the field also suggest that scientists may one day use stem cells to repair muscle tissues following heart attacks and even save the giant panda from extinction.
Embryonic Stem Cells Revolutionized Developmental Biology Research
A better understanding of early embryonic development can help researchers identify the causes of miscarriages and developmental disorders. Mammalian embryos are particularly difficult to study because they develop in the uterus. In vitro fertilization researchers in the 1970s learned how to culture human embryos outside the womb, but international ethics guidelines prevented them from growing embryos beyond 14 days post fertilization. In the 1990s, scientists began to develop new models on human embryonic stem cells (ESCs), which revolutionized the field. Today, ESC-based methods still have a lot of room for improvement to make them more robust, efficient, and reproducible, but they’ve allowed researchers to investigate embryonic development at various stages: pre- or post-implantation, even beyond in the 14 to 21-day time window after fertilization.
The Many Paths to Stem Cell-Based Embryo ModelsFor several years, researchers studied human embryonic stem cells (ESCs) to understand the unique features of these pluripotent cells, but on their own, they poorly resembled the complex structures that they derived from. While human embryos donated from in vitro fertilization clinics offered insights into the early development period, they are technically and ethically challenging to work with. In recent years, scientists developed a variety of stem cell-based models of embryos to overcome these challenges. (1) Embryoid Bodies: In a first example of a human 3D embryo model, researchers grew ESCs on non-adhesive dishes in media without a differentiation inhibitor or growth factor to develop into embryoid bodies (EBs) that have all three embryonic germ layers: ectoderm, mesoderm, and endoderm. EBs aid in drug screening and developing some organoids. (2) Micropattern Colonies: To study the spatial organization of human embryos, researchers treated ESCs with a growth factor and grew them in geometrically confined wells with extracellular matrix coating. These ESCs differentiated and assembled into radial layers of three embryonic germ tissues, or micropattern colonies. Researchers use these spatially organized colonies to study gene expression and the role of cell density in embryo development. (3) Blastoids: In 2021, four groups independently found that inhibiting growth and differentiation factors in naïve or primed pluripotent stem cells (PSCs) and culturing the cells on non-adherent hydrogel generated a blastocyst-like structure, a blastoid, with cells of the trophectoderm, the epiblast, and the primitive endoderm. Blastoids resemble the pre-implantation period of embryo development. (4) Post-Implantation Embryo Models: Scientists differentiated PSCs (naïve or primed stage) into lineages representing the primitive endoderm and trophectoderm and aggregated these with PSCs, modeling the epiblast, in defined ratios. These aggregates assembled into structures modeling the post-implantation embryo—an important milestone in developmental biology that could enable researchers to study this critical stage in the future. (5) Gastruloids: To overcome limitations in studying embryonic development after 14 days, one group pre-treated ESCs with an activator of a proliferation pathway and then cultured the cells at a density of 300 cells in a low-adherence dish, prompting the ESCs to aggregate. Over 96 hours, they saw that these aggregates elongate, mimicking gastrulation, and cells begin to differentiate into those representing all three germ layers. These gastruloids allow scientists to study the earliest period of organ development that is prohibited in embryo research. |

Jorge Silvio Gutkind studies growth-promoting signal transduction pathways in cancer to better understand cancer progression.
Kyles Dykes/University of California San Diego Health Sciences
Hippo Signaling Could Turn Oral Stem Cells Malignant
About thirty percent of oral cancers arose from human papillomavirus (HPV) infection. Researchers commonly study HPV-positive cancer in advanced stages, but they didn’t know much about the earlier events in disease progression, such as how healthy stem cells transform into malignant ones. Recently, molecular biologist Jorge Silvio Gutkind at the University of California, San Diego, and his colleagues, used transcriptomics and microscopy to characterize these processes. The researchers activated the Hippo signaling pathway in mouse models of oral cancer, which caused the mice to develop cancer within two weeks. Gutkind’s team observed significant changes in chromatin accessibility and expression of genes associated with cell proliferation, invasion, and inflammation.
Loss of a Kinase Helps Colorectal Tumors Rise from Dead Stem Cells
Colorectal cancers kill more than 900,000 people each year. In 2018, cancer biologists Maria Diaz-Meco, Jorge Moscat, and their colleagues reported that the loss of atypical protein kinase C (aPKC) isoforms led to tumor growth in mouse intestines. More recently, the researchers discovered an intermediary step that mediates the two phenomena: the death of intestinal stem cells (ISCs). They showed that deletion of the aPKC isoforms reduced ISC population within three days. The manipulation also led to the activation of cell death transcription programs and the appearance of precancerous cells that divide uncontrollably. Understanding the molecular details of colorectal tumor formation could reveal new therapeutic targets to help patients with this aggressive cancer.
Healthy Hair Stem Cells Eat Their Dying Neighbors
Professional phagocytes, such as macrophages and dendritic cells, work full-time as dead and dying cell patrol. But certain parts of the body, like the hair follicles, are off-limits to these cells. To prevent dead cells from accumulating, other cells must take up this task. Recently, stem cell biologists Katherine Stewart, Elaine Fuchs, and their colleagues discovered that the duty fell on hair follicle stem cells. The researchers found that healthy stem cells upregulate the expression of a molecule that help them find and phagocytose their dying neighbors.
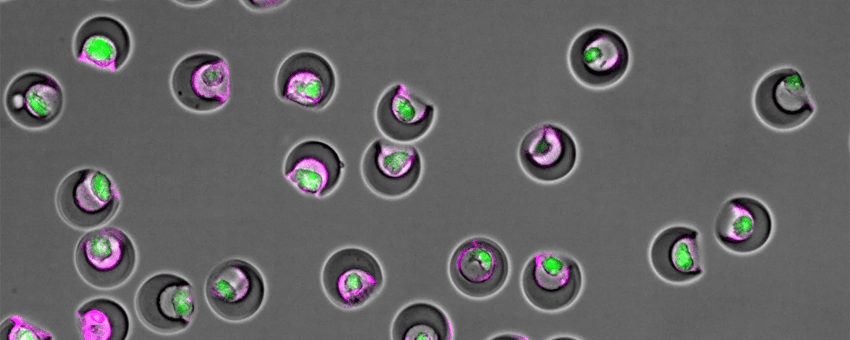
Scientists used nanovials (grey) lined with antibodies to capture single mesenchymal stem cells (green) and measure their secretion of extracellular vesicles (magenta).
Doyeon Koo, UCLA
Productive Stem Cells Could Help Heart Muscles Regenerate
Mesenchymal stem cells (MSCs) are adult stem cells that are typically associated with the bone marrow. They can self-renew and differentiate into multiple cell types: bone, cartilage, muscle, and fat, so scientists hope to use them for regenerative therapies following spinal cord injuries and heart attacks. Researchers believe that MSCs’ magic lies in the compounds they secrete in extracellular vesicles (EVs), but EV production is highly variable, and this makes their translation into the clinic challenging. Recently, bioengineer Dino Di Carlo and his colleagues used microfluidics and nanotechnology to separate high EV-producing MSCs from their less productive work mates. When injected into a mouse model of heart attacks, the high-secreting MSCs promoted higher rates of regeneration and improved heart function compared to their low-secreting counterparts in just 28 days.

Scientists reprogrammed skin cells from giant pandas to create stem cells, which could aid in the conservation of this vulnerable species.
©iStock, DennisvandenElzen
A Special Stem Cell Recipe Could Save the Giant Panda
For nearly over a decade, scientists have been trying to use induced pluripotent stem cells (iPSCs) to rescue species on the brink of extinction, including the northern white rhinoceros and giant pandas. In 2015, a group of researchers tried to generate iPSCs from giant panda cheek cells, but these cells weren’t truly pluripotent. Recently, Jing Liu, a stem cell biologist at the Chinese Academy of Sciences, tried a different cell type: fibroblasts. With the help of a microRNA cluster, panda-specific transcription factors, and a few other experimental tweaks, Liu’s team showed that the induced stem cells, when injected into mice, could form the essential cell layers that define early embryonic development. Liu hopes to use the iPSCs to produce sperm and egg cells that could someday make giant panda babies.