Ticks are eight-legged parasites that can transmit diseases by carrying pathogenic organisms, such as bacteria and viruses. Lyme disease, the most common tick-borne illness, is caused by the bacteria Borrelia burgdorferi and spread through the bite of infected blacklegged ticks. It affects nearly half a million people in the United States each year and can cause neurological and cardiac dysfunction if left untreated. The incidence of tick-borne illnesses typically rises in the summer as people spend more time outdoors, increasing their risk for exposure, and warmer temperatures can help ticks live longer. Read the latest stories about ticks, the harm they bring, and recent breakthroughs in the development of a Lyme disease vaccine.
Ticks Form a “Cement Cone” to Latch onto a Surface
Not all tick bites cause a pathogenic infection, but this risk increases the longer ticks latch onto the skin. While many animals, such as mussels and spiders, can glue themselves to hard surfaces, ticks are unique because they can stick to living things, including human skin. Recently, Siddharth Deshpande, a biophysicist at Wagenigen University and Research, and his colleagues discovered that when ticks fed on blood, a type of ubiquitous protein in their saliva became even more abundant. The upregulation of this protein ultimately caused tick saliva to shape-shift into a solid “cement cone”. Understanding how tick glue forms may one day allow scientists to develop strong tissue sealants that can help wound healing.
Lone Star Ticks Cause Alpha-Gal Syndrome
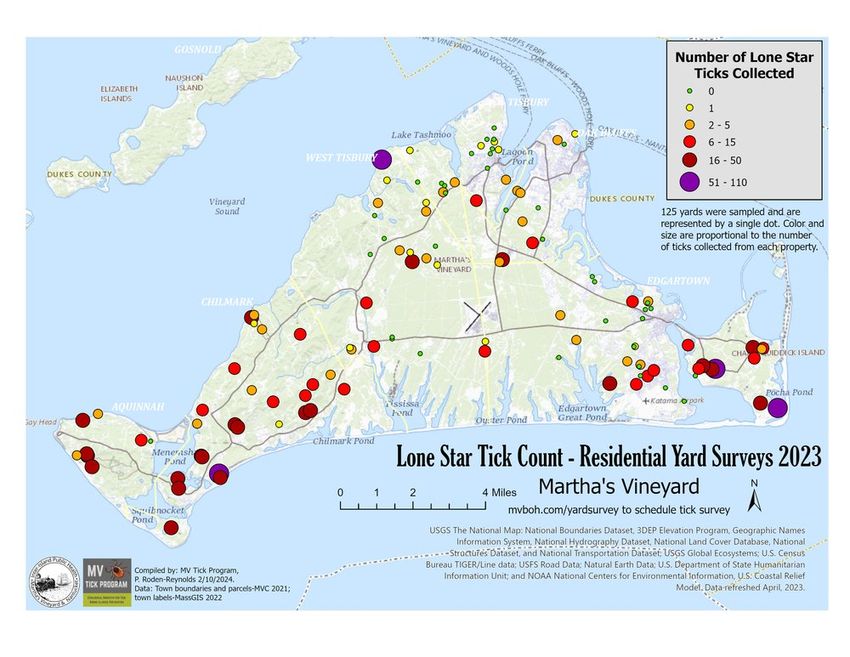
For more than a decade, the Martha’s Vineyard Tick Program has surveyed ticks in residential yards across the island. Over the last few years, the lone star tick population has increased and spread across the island.
Patrick Roden-Reynolds
In the early 2000s, Thomas Platts-Mills, an allergy specialist at the University of Virginia (UVA), was trying to solve why some of his patients suddenly became allergic to red meat. Around the same time, oncologists at his university were trying to solve why an unusually high number of their patients reacted severely to the new cancer antibody drug cetuximab. Platts-Mills and his colleagues soon discovered that antibody against the sugar galactose-α-1,3-galactose, or alpha-gal, caused both allergic reactions. They also found, to their surprise, that many of these patients had a history of tick bites. Platts-Mills and his colleagues, and more recently, Patrick Roden-Reynolds, a public health biologist and director of the Tick-Borne Illness Reduction Initiative in Martha’s Vineyard, Massachusetts, observed a link between the increase in lone star tick population and alpha-gal syndrome incidence.
Ghosts of Tick Infections Past Haunt the Liver
Borrelia burgdorferi can modify molecules in their cell walls with tick sugar, which helps them persist in the liver and cause chronic Lyme disease symptoms.
©iStock, AlexLMX
Physicians commonly prescribe antibiotics to treat Lyme disease, but approximately 15 percent of patients report experiencing fatigue and pain after completing their treatment—this condition is commonly known as post-treatment Lyme disease syndrome (PTLDS). In 2019, microbiologist Brandon Jutras, then at Yale University, and his team discovered a cell wall component of B. burgdorferi, the Lyme disease-causing bacteria, in the inflamed joints of patients affected with PTLDS, but they were puzzled by how the molecules could persist for so long. More recently, Jutras’s team found that B. burgdorferi could alter this cell wall component using a tick sugar, and this modification allowed the molecule to stay longer in mouse liver.
Development of Lyme Disease Vaccines

Nobel Prize winner Drew Weismann, then graduate student Matthew Pine, and their colleagues developed an mRNA-based vaccine against Lyme disease.
Rebecca Dougherty
In 2023, Drew Weismann and Katalin Karikó were awarded the Nobel Prize in Physiology or Medicine for their contribution to the development of mRNA vaccines against COVID-19. Recently, Weismann and his colleagues developed an mRNA-based vaccine against Lyme disease which targets B. burgdorferi’s outer surface protein (OspA). Weismann’s team, led by then graduate student Matthew Pine, showed that in mice, the vaccine enhanced the production of long-lived antibody-producing cells and activation of the adaptive immune response upon re-exposure.
Recently, a different group of researchers, led by Yi-Pin Lin, an infectious disease biologist at Tufts University, put forward another Lyme disease vaccine candidate: an engineered version of a B. burgdorferi protein. The complement regulator-acquiring surface protein 2 (CspZ) can help B. burgdorferi hide from the immune system, but Lin and his colleagues previously discovered that they could get rid of this immune evasion ability by mutating two amino acids on the protein. Recently, Lin’s team mutated two more amino acids on CspZ to increase its stability, an important quality for vaccine candidates, and found that mice that were immunized with this engineered CspZ produced higher titers of antibody and showed less severe Lyme disease symptoms.













