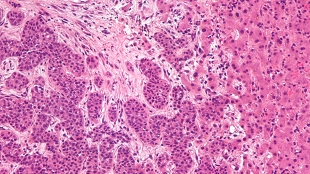
 Liver metastasis. Wikimedia, Nephron.A literal window into the insides of mice is giving scientists a peek at the accumulation of cancer cells in organs deep within the body. Watching through a transparent coverslip surgically implanted into the animals’ abdominal cavities, researchers in The Netherlands identified a transition that takes place as cancer cells go from a mobile state in which they infect new organs to a non-migratory state that allow the cells to multiply and establish new tumors. The new technique, published today (October 31) in Science Translational Medicine, will help shed light on the important early steps of metastasis and possibly enable to the development and screening of metastases-targeted drugs.
Liver metastasis. Wikimedia, Nephron.A literal window into the insides of mice is giving scientists a peek at the accumulation of cancer cells in organs deep within the body. Watching through a transparent coverslip surgically implanted into the animals’ abdominal cavities, researchers in The Netherlands identified a transition that takes place as cancer cells go from a mobile state in which they infect new organs to a non-migratory state that allow the cells to multiply and establish new tumors. The new technique, published today (October 31) in Science Translational Medicine, will help shed light on the important early steps of metastasis and possibly enable to the development and screening of metastases-targeted drugs.
“Metastasis is the big question in cancer right now, and so little is known of the early and late steps,” said Zena Werb, a molecular biologist at the University of California, San Francisco, who did not participate in the study. The initial movement of cells metastasizing to the liver is “not unexpected, but it’s the first time it’s been seen,” said Werb.
“Metastatic sites that are most interesting are deep within the body, making it hard to find out what’s happening at that stage when metastases are 2, 3, 4 cells in size,” explained Erik Sahai, a tumor cell biologist at the Cancer Research UK London Research Institute, who did not participate in the study. A window into a highly-metastasized organ, like the liver, gives ...



















