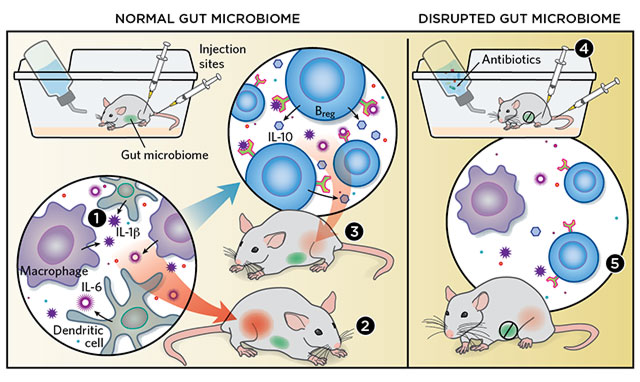
 MICROBIAL INSTIGATORS: Conventionally housed mice are exposed to ample amounts of bacteria, which colonize their guts. When researchers induce arthritis in the mice by injecting them with foreign antigens, the animals’ macrophages and dendritic cells go into overdrive, producing the cytokines IL-1β and IL-6 (1). These cytokines lead to acute arthritis (2). But they also drive the development of Bregs, which produce the anti-inflammatory cytokine IL-10 that subsequently quells arthritic symptoms (3). Meanwhile, mice raised in a sterile environment or given antibiotics to knock down their gut microbiota (4) suffer less acute arthritis, due to a relative lack of IL-1β, IL-6, and other pro-inflammatory cytokines. They also produce fewer Bregs (5). © KIMBERLY BATTISTA
MICROBIAL INSTIGATORS: Conventionally housed mice are exposed to ample amounts of bacteria, which colonize their guts. When researchers induce arthritis in the mice by injecting them with foreign antigens, the animals’ macrophages and dendritic cells go into overdrive, producing the cytokines IL-1β and IL-6 (1). These cytokines lead to acute arthritis (2). But they also drive the development of Bregs, which produce the anti-inflammatory cytokine IL-10 that subsequently quells arthritic symptoms (3). Meanwhile, mice raised in a sterile environment or given antibiotics to knock down their gut microbiota (4) suffer less acute arthritis, due to a relative lack of IL-1β, IL-6, and other pro-inflammatory cytokines. They also produce fewer Bregs (5). © KIMBERLY BATTISTA
The paper
E.C. Rosser et al., “Regulatory B cells are induced by gut microbiota–driven interleukin-1β and interleukin-6 production,” Nat Med, 20:1334-39, 2014.
Elizabeth Rosser was partway through her PhD at University College London when her experiments stopped working. Using a mouse model of arthritis, she was trying to understand how invariant natural killer T cells—white blood cells that help guide the body’s response to inflammation—interact with regulatory B cells (Bregs), which put a damper on inflammatory responses. But when the university’s Centre for Rheumatology moved to a new building, experimental mice didn’t get arthritis—and also didn’t develop Bregs.
It is well-known that animals’ immune systems do not always behave the same way in new, very clean housing facilities as in older ones. The literature shows ...


















