A trip to the grocery store is a sensory adventure, with aisles brimming with eye-catching packages designed to tempt shoppers. Each display promises a delicious and memorable food adventure. “We’ve all experienced this moment where we crave a specific food, even if we’re not physically hungry,” said Guillaume de Lartigue, a neuroscientist at the Monell Chemical Senses Center, who studies how the brain controls food intake.
Even a single whiff of fresh bread from a nearby bakery can evoke mouthwatering memories, instantly sparking hunger. This connection between memory and appetite led de Lartigue to wonder how memory centers in the brain influenced eating behavior and whether they contributed to obesity risk.

Guillaume de Lartigue is fascinated by the neurobiology of feeding.
Guillaume de Lartigue
In a new study, published in Nature Metabolism, de Lartigue and his team identified two distinct populations of hippocampal neurons that responded to either the sugar or fat content of a recently-consumed meal.1 Activating these neurons strengthened contextual memory and motivation in mice, helping them locate and consume these nutrients. Conversely, silencing either population impaired the recall of food-related memories and reduced the intake of high-calorie foods, preventing excessive weight gain. Understanding how the brain’s food-specific memory system influences eating habits could pave the way for developing new obesity treatments.
These findings uncovered previously unknown neurons that promote eating behavior, shedding light on the neural mechanisms that could contribute to disordered eating behaviors, such as overeating. “If you want to understand disordered eating, you have to [first] understand how eating works,” remarked Marise Parent, a neuroscientist at Georgia State University who was not involved in the study. “[This study addresses] this big gap of knowledge.”
Eating triggers the gut to send information about the meal’s nutritional contents and fullness to the brain, helping regulate appetite. Previous studies showed hippocampal activation after a mixed-nutrient meal, but whether specific neurons respond to individual nutrients was unclear.2
Given de Lartigue’s interest in the neurons’ role in obesity, the team focused on how the hippocampus responded to macronutrients associated with diet-induced obesity. They predicted that a single group of neurons would broadly respond to the consumption of both fat and sugar. The researchers gave mice intragastric infusions of saline, sugar, or fat solutions. Then, after 90 minutes, they removed the brains and measured Fos protein, a marker of neuronal activity.
Instead of neurons that responded to both sugar and fat, de Lartigue was surprised to find that Fos levels increased in two separate neuronal populations in the dorsal hippocampus (dHPC) of mice that received sugar or fat. Even when mice received sugar and fat infusions two weeks apart, the pattern was consistent, suggesting that there were two spatially distinct, nutrient-specific groups of neurons in the dHPC.
Since these neurons were activated after a meal, de Lartigue investigated whether the vagus nerve, a crucial gut-brain pathway, played a role in their activation. The vagus nerve detects nutrient signals and hormonal changes in the gut, relays this information to the brain, and can influence hippocampal activity. Indeed, when they surgically impaired the vagus nerve and then administered saline, sugar, or fat, Fos expression was significantly reduced compared to control mice.
“[This] suggests that the brain is actually keeping track of what we are eating,” remarked de Lartigue. He explained that he assumed the brain was only tracking calories, and it didn’t matter what the source of food was.
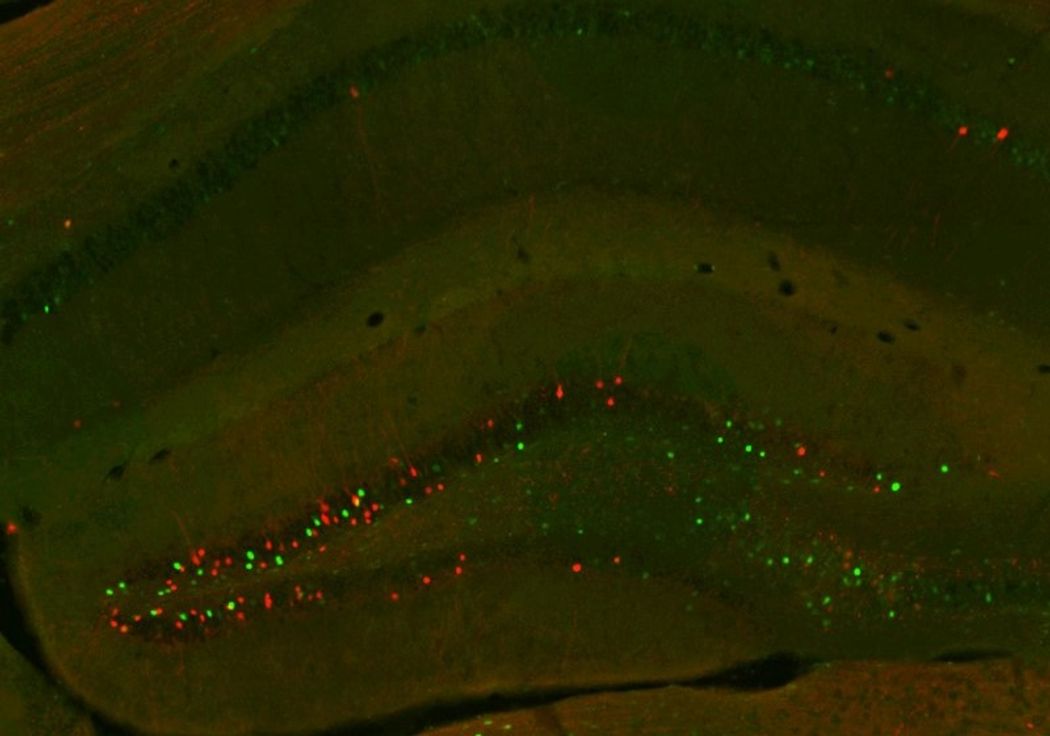
Researchers found two distinct populations of neurons in mouse hippocampus that recognize fat (green) or sugar (red).
Mingxin Yang
Next, de Lartigue wanted to understand how these dHPC neurons were linked to eating behavior and whether they drove preferences for sugar or fat. If the brain forms positive memories of food, it could drive the continuous desire for calorie-dense foods. First, the researchers compared control mice to those with silenced sugar- or fat-responsive neurons. They provided all three groups with two equicaloric bottles—one with a sugar solution, the other with fat. Over a three-day testing period, control mice preferred the fat solution, while mice with ablated sugar- or fat-responsive neurons consumed 40–50 percent less of their respective nutrients, with no impact on the intake of the other.
After determining that these neurons were macronutrient specific, de Lartigue sought to understand how these neurons affected memory and motivation, particularly in recalling the location of sugars or fats and driving the consumption of these nutrients. “To our even bigger surprise, these two populations do different things,” he remarked.
In a food-cup location memory task, mice learned to find a nutrient-containing dish. Control mice remembered both sugar and fat locations, but those with silenced sugar- and fat-responsive neurons showed impaired memory for their respective nutrients.
The researchers found that stimulating sugar neurons helped mice remember sugar placement but did not affect fat memory, suggesting sugar-responsive neurons are involved in forming sugar-related memories.
What about fat-responsive neurons? Rather than remembering the location of a nutrient, these neurons might play a role in motivation and reward instead. To test this, the researchers measured how much effort mice were willing to exert, by licking from a dry sipper bottle, to obtain small amounts of sugar or fat. While mice with silenced sugar neurons showed no change in this behavior for sugar compared to control mice, mice with silenced fat-responsive neurons licked less for fat, suggesting these neurons drove fat-specific motivation.
The researchers then explored how these neurons regulate food intake on a high-fat, high-sugar diet. Silencing either neuron type reduced the intake of its respective macronutrient but through distinct mechanisms—sugar neurons affected memory, while fat neurons influenced motivation. This reduction ultimately prevented weight gain, providing protection against diet-induced obesity.
“I was tremendously impressed by their finding that different neurons sense different macronutrients. I underestimated the level of sophistication in hippocampus involvement,” said Parent.
De Lartigue remarked that there is some evidence of similar results in humans, where the hippocampus is activated by food cues, specifically in individuals who are obese.3 Given these parallels in food memory circuits, he emphasized the importance of identifying the molecular profiles of hippocampal neurons to determine the receptors they express, which could inform the development of targeted drugs to modulate their activity. These findings provide new insight into developing novel strategies to combat overeating and obesity by disrupting memory-driven cravings for unhealthy, calorie-dense foods.
- Yang M, et al. Separate orexigenic hippocampal ensembles shape dietary choice by enhancing contextual memory and motivation. Nat Metab. 2025;7:276-296.
- Min DK, et al. Changes in differential functional magnetic resonance signals in the rodent brain elicited by mixed-nutrient or protein-enriched meals. Gastroenterology. 2011;141(5):1832-1841.
- Barbosa DAN, et al. An orexigenic subnetwork within the human hippocampus. Nature. 2023;621(7978):381-388.















