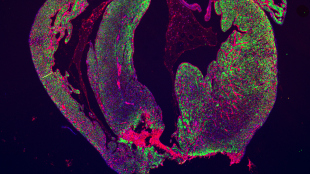
 The resected area of a mouse heart is still missing and scar formation (red) is seen in the border of the resection line.STEM CELL REPORTS, ANDERSEN ET AL.In 2011, researchers reported having successfully induced the hearts of newborn mice to regenerate after a surgery. The feat was a major accomplishment in demonstrating the ability of a mammalian heart to regenerate, and the publication has been cited more than 300 times. But in a study published today (April 3) in Stem Cell Reports, a separate team tested the protocol and could not reproduce the findings.
The resected area of a mouse heart is still missing and scar formation (red) is seen in the border of the resection line.STEM CELL REPORTS, ANDERSEN ET AL.In 2011, researchers reported having successfully induced the hearts of newborn mice to regenerate after a surgery. The feat was a major accomplishment in demonstrating the ability of a mammalian heart to regenerate, and the publication has been cited more than 300 times. But in a study published today (April 3) in Stem Cell Reports, a separate team tested the protocol and could not reproduce the findings.
“I haven’t seen one heart that has been regenerated in our lab,” said Ditte Andersen, the lead author of the new study and a researcher at the University of Southern Denmark. Andersen told The Scientist that she doesn’t have an explanation for the discrepancy. “We’ve discussed everything,” she said, but her team could not find a reason.
Hesham Sadek, the senior author of the 2011 paper and a researcher at the University of Texas Southwestern Medical Center in Dallas, read Andersen’s report and said the problem is technical. “I think that their techniques are flawed,” he said, although he would not provide further detail.
Sadek’s procedure involved cutting out about 15 percent of the heart at ...














