 TINY pH DETECTORS: The team encapsulated live liver cells together with a liposome containing L-arginine inside a hydrogel microcapsule before transplantation into mice.GEORGE RETSECK
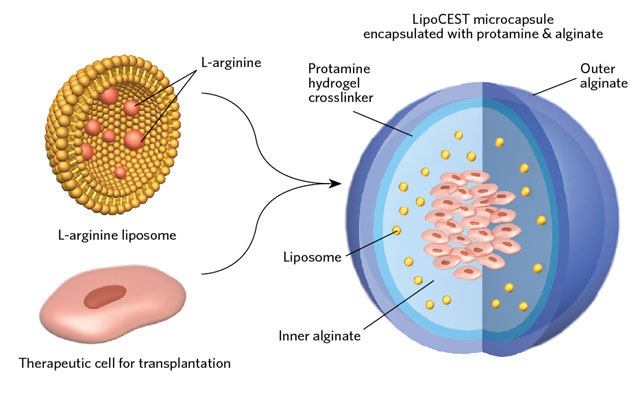
TINY pH DETECTORS: The team encapsulated live liver cells together with a liposome containing L-arginine inside a hydrogel microcapsule before transplantation into mice.GEORGE RETSECK MONITORING CELL VIABILITY BY MRI: When cells die, the acidity within the microcapsule increases, changing the contrast of the L-arginine liposomes. Using CEST-MRI the researchers estimated a 33 percent drop of signal when all of the 2 million transplanted cells died. GEORGE RETSECK; MRI IMAGES COURTESY OF MICHAEL MCMAHONEncapsulating therapeutic cells inside hydrogel microparticles before transplanting them into the body has become a promising approach for cell-based therapies, such as injecting new pancreatic islet cells into diabetic patients whose own cells have stopped producing insulin. The hydrogel not only keeps the cells at the injection site, it spares them from attack by the host’s immune system.
MONITORING CELL VIABILITY BY MRI: When cells die, the acidity within the microcapsule increases, changing the contrast of the L-arginine liposomes. Using CEST-MRI the researchers estimated a 33 percent drop of signal when all of the 2 million transplanted cells died. GEORGE RETSECK; MRI IMAGES COURTESY OF MICHAEL MCMAHONEncapsulating therapeutic cells inside hydrogel microparticles before transplanting them into the body has become a promising approach for cell-based therapies, such as injecting new pancreatic islet cells into diabetic patients whose own cells have stopped producing insulin. The hydrogel not only keeps the cells at the injection site, it spares them from attack by the host’s immune system.
One hindrance to the technique’s more widespread clinical application, however, has been the inability to safely assess the cells’ long-term survival and function once implanted. Michael McMahon at Johns Hopkins University in Baltimore, Maryland, and colleagues have now created biocompatible cell-viability nanosensors that can be incorporated into the hydrogel.
McMahon’s team had previously shown that L-arginine is an excellent contrast agent—a substance that enhances visualization—for a version of MRI called chemical exchange saturation transfer (CEST). The degree of contrast exhibited by the chemical depends on the pH of its surrounding environment. So McMahon devised a sensor out of lipid nanoparticles that contained L-arginine.
The team tested the particles’ cell death–detection ability by encapsulating them, along with liver cells, in hydrogel microcapsules, and transplanting them into ...



















