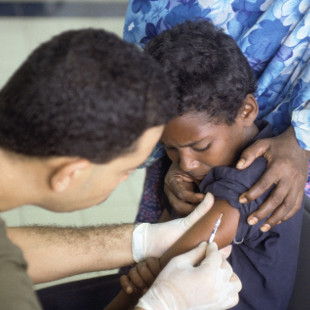
 WIKIMEDIA, ANDREW W. MCGALLIARDDepending on when and where in the world you were born, you may have received a different kind of polio vaccine than someone else born into different circumstances. If you were born in the U.S. after 2000, for example, you likely received an injection of the inactivated polio vaccine (IPV), but before that, you might have received the oral polio vaccine (OPV), a live attenuated virus, administered by mouth. Starting later this year, children born in polio-affected countries will receive both vaccine types, per World Health Organization (WHO) “endgame” plans to finish the job of eradicating polio.
WIKIMEDIA, ANDREW W. MCGALLIARDDepending on when and where in the world you were born, you may have received a different kind of polio vaccine than someone else born into different circumstances. If you were born in the U.S. after 2000, for example, you likely received an injection of the inactivated polio vaccine (IPV), but before that, you might have received the oral polio vaccine (OPV), a live attenuated virus, administered by mouth. Starting later this year, children born in polio-affected countries will receive both vaccine types, per World Health Organization (WHO) “endgame” plans to finish the job of eradicating polio.
The results of a study published today (August 21) in Science support this approach. In a group of Northern Indian children who had already received one or more doses of OPV, a supplementary dose of IPV bolstered their immune responses and reduced their shedding of viral particles in stool, a team led by researchers at the WHO reported.
Since 1988, more than 10 billion doses of OPV have been administered and more than 10 million polio cases have been prevented, according to the WHO. However, the intestinal or mucosal immunity proffered by the vaccine, which in an ideal situation both prevents virus from surviving within the gut and from spreading to the nervous system, wanes after as little as six months. And booster doses of ...




















