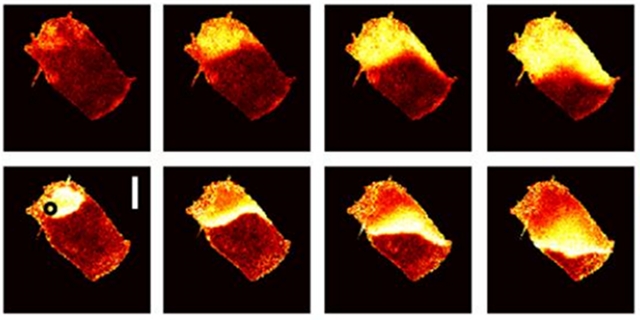
 Calcium (top) and voltage (bottom) flux over time in rat cardiac tissue slices. PETER LEE, CHRISTIAN BOLLENSDORFF
Calcium (top) and voltage (bottom) flux over time in rat cardiac tissue slices. PETER LEE, CHRISTIAN BOLLENSDORFF
THE DEVICE: A complex interplay of signals governs the heart’s rhythm. Voltage changes and calcium flux are both important in controlling heart muscle function, with each signal influencing the other’s dynamics. Scientists at the University of Oxford have created a single camera system that can capture the dynamics of these signals simultaneously, yielding important insight into their relationship.
Peter Lee and colleagues combined several colors of light emitting diodes (LEDs) with a multi-band emission filter so that one very high speed camera could capture the different wavelengths of light emitted by various fluorescent dyes. By using different colors of LEDs, they were able to stimulate different dyes to measure changes in calcium and voltage across cardiac tissue or single layers of human cardiomyocytes (created from ...




















