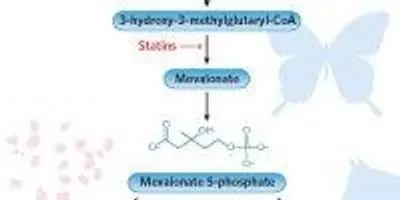
CHARTING THE COURSE: Eukaryotes use the classic mevalonate (MVA) pathway (blue) to produce isopentenyl diphosphate (IPP), a precursor molecule necessary for cholesterol biosynthesis. The discovery of the enzymes isopentenyl phosphate kinase (IPK) and, most recently, mevalonate phosphate decarboxylase (MPD) chart an alternate route for at least some bacteria and archaea. Statin drugs limit cholesterol production by interfering with IPP production. Abbreviations: phosphomevalonate kinase (PMK), diphosphomevalonate decarboxylase (MDD)MODIFIED FROM eLIFE, doi:10.7554/eLife.00672.002, 2013
CHARTING THE COURSE: Eukaryotes use the classic mevalonate (MVA) pathway (blue) to produce isopentenyl diphosphate (IPP), a precursor molecule necessary for cholesterol biosynthesis. The discovery of the enzymes isopentenyl phosphate kinase (IPK) and, most recently, mevalonate phosphate decarboxylase (MPD) chart an alternate route for at least some bacteria and archaea. Statin drugs limit cholesterol production by interfering with IPP production. Abbreviations: phosphomevalonate kinase (PMK), diphosphomevalonate decarboxylase (MDD)MODIFIED FROM eLIFE, doi:10.7554/eLife.00672.002, 2013
The paper
N. Dellas et al., “Discovery of a metabolic alternative to the classical mevalonate pathway,” eLife, doi:10.7554/eLife.00672.002, 2013.
The first steps in the biosynthesis of cholesterol are collectively called the mevalonate (MVA) pathway and result in the generation of isopentenyl diphosphate (IPP). The synthesis of IPP is essential in all organisms as a starting point for the formation of isoprenoids—a large group of compounds that include cholesterol, hormones, and signaling molecules, and that chemists have tapped for vitamins, fragrances, flavorings, and pigments, among hundreds of other uses. Statin drugs target the MVA pathway and limit the output of IPP to lower cholesterol levels.
Bacteria, archaea, and eukaryotes take different tacks to produce IPP. Eukaryotes generally use the classic five-step MVA pathway, while plant chloroplasts ...