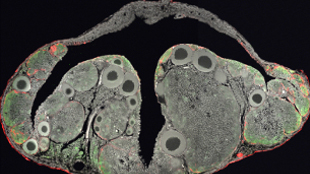
 Foxl3 -/- medaka ovary with both oocytes (large round ovals) and sperm (smaller dots). Black T-shape is cavity into which matured oocytes are ovulated.TOSHIYA NISHIMURA, MINORU TANAKA, NATIONAL INSTITUTE FOR BASIC BIOLOGY, OKAZAKI, JAPANSomatic cells in the gonads of a developing vertebrate provide germ cells with cues, such as hormones, to develop into sperm or eggs. Studying the ways these cues affect a germ cell’s commitment to become sperm or eggs, Toshiya Nishimura from the laboratory of Minoru Tanaka at the National Institute for Basic Biology in Okazaki, Japan, and colleagues uncovered a single gene that, when missing from female embryos of the Japanese rice fish, or medaka (Oryzias latipes), leads the fish to produce functional sperm soon after hatching. The team’s results, published today (June 11) in Science, suggest that—at least in medaka—spermatogenesis must be suppressed for oogenesis to proceed normally, and that sperm can develop in an ovarian environment.
Foxl3 -/- medaka ovary with both oocytes (large round ovals) and sperm (smaller dots). Black T-shape is cavity into which matured oocytes are ovulated.TOSHIYA NISHIMURA, MINORU TANAKA, NATIONAL INSTITUTE FOR BASIC BIOLOGY, OKAZAKI, JAPANSomatic cells in the gonads of a developing vertebrate provide germ cells with cues, such as hormones, to develop into sperm or eggs. Studying the ways these cues affect a germ cell’s commitment to become sperm or eggs, Toshiya Nishimura from the laboratory of Minoru Tanaka at the National Institute for Basic Biology in Okazaki, Japan, and colleagues uncovered a single gene that, when missing from female embryos of the Japanese rice fish, or medaka (Oryzias latipes), leads the fish to produce functional sperm soon after hatching. The team’s results, published today (June 11) in Science, suggest that—at least in medaka—spermatogenesis must be suppressed for oogenesis to proceed normally, and that sperm can develop in an ovarian environment.
“This is exciting because it is absolutely unexpected,” said Manfred Schartl, who studies gonadal development at the University of Würzburg in Germany and was not involved in the current study. “That these germ cells have genetically determined sexual fate is new.”
The gene, foxl3, is an ancient lineage duplicate of the foxl2 gene, which is known to be involved in mammalian ovary formation. But the function of foxl3 was previously unclear. “In mammals, foxl2 is well known to antagonize male somatic cell fate and now foxl3, at least in medaka, seems to antagonize male germ cell fate,” said Josephine Bowles, a senior research fellow at the University of Queensland in Australia who studies sex determination in mammals and was not involved in the study.
The ...


















