While amyloid β plaques are hallmarks of Alzheimer’s disease (AD), recent research suggests that they aren’t the only players in the neurodegenerative disorder. Genome-wide association studies and RNA sequencing data indicate that microglia, the Pac-Man-like cells of the brain that clean up debris and prune unnecessary neurons, are also important.1,2
Gaurav Chopra, a neuroscientist and immunologist at Purdue University, and his team wanted to know how amyloid β affects microglia. While microglia are initially critical in isolating and removing these plaques, sustained inflammatory activation promotes cell damage, and the microglia lose their ability to phagocytose the plaques with age.3,4 However, the mechanisms underlying these effects are poorly understood.
In a study published in Immunity, Chopra and his team showed that amyloid β plaques induce accumulation of lipid droplets in microglia, which leads to their reduced phagocytic function and activity in plaque clearance.5 These findings shed light on the role of lipid metabolism in microglia function during AD and offer potential new routes for therapeutic targeting.
Using a mouse model of AD, the researchers first compared the protein and lipid content in microglia between AD and wild type (WT) mice at early (two to three months) and late (five to seven months) time points. Although older male AD mice showed some increases in lipid content compared to their WT counterparts, the researchers saw that only the five- to seven-month-old female mice with AD had significantly increased lipid content in their microglia compared to age-matched WT female mice. They concluded that AD caused age- and sex-specific effects on lipid accumulation in microglia.

Priya Prakash (left) and Palak Manchanda (middle) studied the effect of amyloid β on microglia as graduate students in Gaurav Chopra’s lab (Chopra, right) at Purdue University.
Steve Scherer
When they screened these lipids with mass spectrometry, they identified 105 mostly downregulated lipids in the female AD microglia compared to female WT microglia, though triacylglycerol species, which form lipid droplet cores, were increased. “That got us to thinking like, ‘So what's the connection between amyloid and lipid droplets in microglia?’” said Priya Prakash, a study coauthor and currently a postdoctoral researcher at New York University.
Previous research showed that many genes involved in lipid metabolism are risk factors for AD.6 Additionally, extended inflammatory responses increase droplet accumulation, in particular in microglia.7 While researchers have studied lipid droplet accumulation extensively in disorders in other organs, “in the brain, it was not really very well known what the role of lipid accumulation is,” Chopra said.
To start to answer this question, Chopra and his team studied the distribution of lipid-rich microglia in the mouse brain. They saw increased lipid droplets in the hippocampus of AD brains, and the density of these cells with these droplets correlated with plaque density. The team observed similar patterns in human brains.
Seeing this relationship between lipid droplets and amyloid β plaques, the researchers investigated whether these factors altered the microglial function. When they treated isolated microglia with amyloid β, the lipid droplet content only increased in microglia from WT mice, not those from AD mice. However, using a fluorescent probe to track amyloid β entry into lysosomes, the team showed that microglia from AD mice had decreased phagocytic activity, suggesting that long-term exposure to amyloid β plaques causes detrimental changes to microglia function.
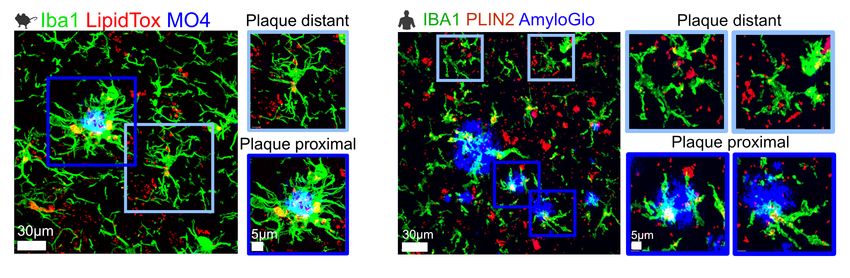
Amyloid β plaques induce lipid buildup in microglia in both mouse models of Alzheimer’s disease and the brains of people who had the disease.
Chopra Lab
“That kind of supports the idea that maybe the amyloid β at the plaque is a factor. I would say it doesn't exclude also the possibility of changes in inflammation and reactive oxygen species formation at the site of plaque, but it could maybe indicate that the [amyloid β], by itself, is important,” said Nicholas Fitz, a neuroscientist at the University of Pittsburgh who was not involved in the study. However, he added, “Is [lipid droplet accumulation] linked to the beginning of the progression of the pathology and facilitates the progression, or is this an end stage phenotype that is seen later on when you have a lot of damage already occurring?”
To further study how amyloid β affects microglia’s metabolic state, the researchers isolated microglia from WT mice and exposed them to amyloid β. One hour after exposure, they observed increased long chain free fatty acid content. By 24 hours, though, the lipid profile shifted so that triglycerides were the most upregulated species.
Considering the enzymatic pathways involved in triglyceride synthesis, the team focused on diacylglycerol O-acyltransferase 2 (DGAT2), an enzyme in the last rate-limiting step in the triacylglycerol pathway. They saw that this protein was increased in the microglia nearest amyloid β plaques in both mice and humans with AD.
“DGAT2 is a really special protein, not because we published it, but because it’s actually not accumulated in healthy tissue to a large extent,” Chopra said. He continued, “This gives a really important angle for drug discovery as well.”
Exploring DGAT2’s potential as a drug target, the researchers treated microglia in vitro with a DGAT2 inhibitor. Although they saw a decrease in overall lipid content in both WT and AD cells, after treating the microglia with amyloid β, the inhibitor only continued to decrease the lipid droplet content in WT cells, whereas AD microglia lipid content was unaffected. However, this treatment increased phagocytic function in AD microglia, illustrated by increased amyloid β entering the lysosome.
Seeing these microglial responses in vitro, they turned to mice to study the effects of degrading DGAT2. After one week of treatment, this degrader reduced the amount of amyloid β plaque and lipid droplet content in the microglia of AD mice. It also reduced neuronal damage as indicated by a decrease in a marker for degenerated axons.
Rada Koldamova, a translational neuroscientist at the University of Pittsburgh who was not involved in the study, also studies the role of lipid metabolism in Alzheimer’s disease. Along with Fitz, her group previously showed that some lipids aid in the transport of amyloid β to microglia to promote plaque clearance. “We cannot be 100 percent sure that these changes that are observed and noted here are not a result of the increased function of microglia [and] the stress upon it to phagocytose amyloid,” she said.
Chopra’s team is currently exploring the effect of these accumulated lipids on neurons and the immune system in the brain as well as how the composition of the lipid droplets influences disease pathology.
- Hollingworth P, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nat Genet. 2011;43(5):429-435.
- Keren-Shaul H, et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell. 2017;169(7):1276-1290.E17.
- Heneka MT, et al. Neuroinflammation in Alzheimer's disease. Lancet Neurol. 2015;14(4):388-405.
- Krabbe G, et al. Functional impairment of microglia coincides with beta-amyloid deposition in mice with Alzheimer-like pathology. PLoS ONE. 2013;8(4):e60921.
- Prakash P, et al. Amyloid-β induces lipid droplet-mediated microglial dysfunction via the enzyme DGAT2 in Alzheimer’s disease. Immunity. 2025;58(6):1536-1552.e8.
- Fitz NF, et al. Phospholipids of APOE lipoproteins activate microglia in an isoform-specific manner in preclinical models of Alzheimer’s disease. Nat Commun. 2021;12(1):3416.
- Marschallinger J, et al. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat Neurosci. 2020;23(2):194-208.