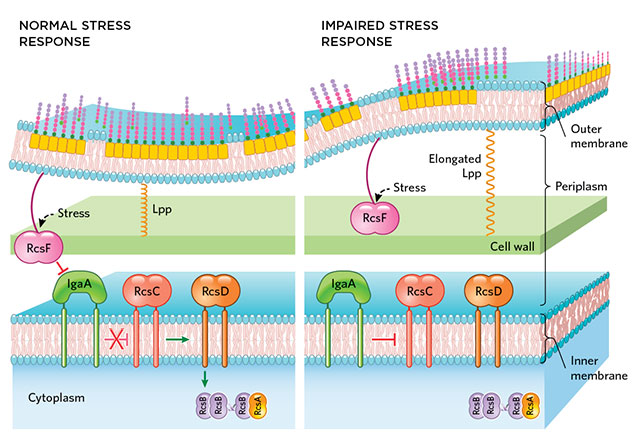
 SIZE MATTERS: When a bacterium encounters a stressor, RcsF inhibits IgaA, lifting its blockage on the activity of downstream components of the Rcs network. But if the periplasmic distance widens, RcsF is unable to reach IgaA, preventing the system from initiating a stress response.© STEVE GRAEPEL
SIZE MATTERS: When a bacterium encounters a stressor, RcsF inhibits IgaA, lifting its blockage on the activity of downstream components of the Rcs network. But if the periplasmic distance widens, RcsF is unable to reach IgaA, preventing the system from initiating a stress response.© STEVE GRAEPEL
The paper
A.T. Asmar et al., “Communication across the bacterial cell envelope depends on the size of the periplasm,” PLOS Biol, 15:e2004303, 2017.
The cell envelope of a gram-negative bacterium protects it from its surroundings and aids survival in another key way: relaying stress signals. “A bacterium like E. coli has several systems that are used to sense stress in the cell envelope,” says Jean-François Collet, a microbiologist at the de Duve Institute in Belgium. One of these, the regulator of capsule synthesis (Rcs) system, depends on the width of the periplasm—the space between the envelope’s inner and outer layers—to function, according to a new study.
The Rcs system senses damage to the cell envelope and responds by modifying the expression of genes involved in ...



















