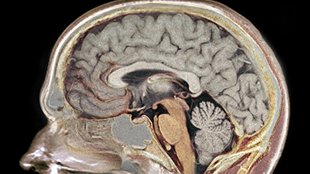
 WIKIMEDIA, GARPENHOLMGenomic analyses of single human neurons—either from postmortem brains or those derived in culture—reveal a considerable degree of DNA copy number variation, according to a paper published today (October 31) in Science. It is likely that these genetic differences affect brain cell function, and they may even shape our personalities, academic abilities, and susceptibilities to neurological diseases.
WIKIMEDIA, GARPENHOLMGenomic analyses of single human neurons—either from postmortem brains or those derived in culture—reveal a considerable degree of DNA copy number variation, according to a paper published today (October 31) in Science. It is likely that these genetic differences affect brain cell function, and they may even shape our personalities, academic abilities, and susceptibilities to neurological diseases.
“It’s an exciting paper. It’s a closer look at the single cell genomes of neurons . . . and it identifies another layer of genomic mosaic changes that are occurring amongst neurons,” said Jerold Chun, a professor of molecular and cellular neuroscience at The Scripps Research Institute in La Jolla, California, who was not involved in the work.
The other genetic changes in neurons, to which Chun referred, are aneuploidy—changes in chromosome number—retrotransposition events—replications of short DNA elements that insert themselves across the genome—and the expression of DNA-altering enzymes, all of which are particularly abundant in the brain.
Ira Hall, a professor of biochemistry and molecular genetics at the University of Virginia who was one of the lead authors on the new study, was interested ...




















