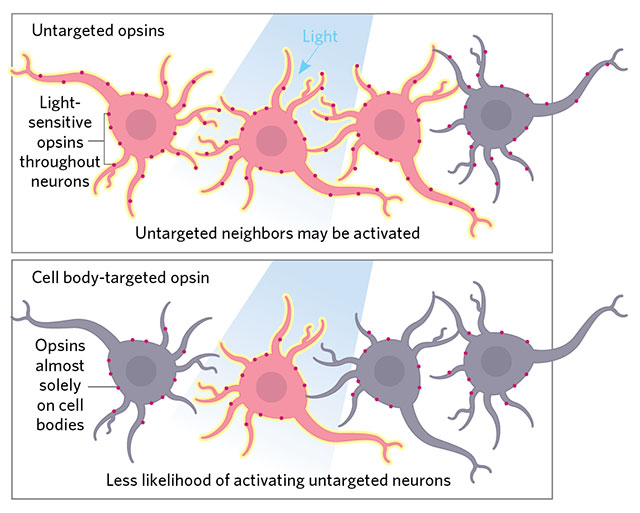
 ON TARGET: Precision illumination techniques target light to individual neuronal cell bodies, but neighboring cells may be activated if their dendrites or axons lie nearby. Unlike regular opsins, which are distributed throughout the entire neuron, cell body–localized opsins, such as those described by Christopher Baker of the Max Planck Florida Institute for Neuroscience (eLife, 5:e14193, 2016) and now Edward Boyden’s team (Nat Neurosci, 20:1796–1806, 2017), prevent such stray activation. Boyden and his collaborators also use a more responsive channelrhodopsin for high-speed firing.© LUCY READING-IKKANDA
ON TARGET: Precision illumination techniques target light to individual neuronal cell bodies, but neighboring cells may be activated if their dendrites or axons lie nearby. Unlike regular opsins, which are distributed throughout the entire neuron, cell body–localized opsins, such as those described by Christopher Baker of the Max Planck Florida Institute for Neuroscience (eLife, 5:e14193, 2016) and now Edward Boyden’s team (Nat Neurosci, 20:1796–1806, 2017), prevent such stray activation. Boyden and his collaborators also use a more responsive channelrhodopsin for high-speed firing.© LUCY READING-IKKANDA
When optogenetics burst onto the scene a little over a decade ago, it added a powerful tool to neuroscientists’ arsenal. Instead of merely correlating recorded brain activity with behaviors, researchers could control the cell types of their choosing to produce specific outcomes. Light-sensitive ion channels (opsins) inserted into the cells allow neuronal activity to be controlled by the flick of a switch.
Nevertheless, MIT’s Edward Boyden says more precision is needed. Previous approaches achieved temporal resolution in the tens of milliseconds, making them a somewhat blunt instrument for controlling neurons’ millisecond-fast firings. In addition, most optogenetics experiments have involved “activation or silencing of a whole set of neurons,” he says. “But the problem is the brain doesn’t work that way.” When a cell is performing ...




















