 Infographic: Smashing Crystals View full size JPG | PDF GEORGE RETSECK
Infographic: Smashing Crystals View full size JPG | PDF GEORGE RETSECK
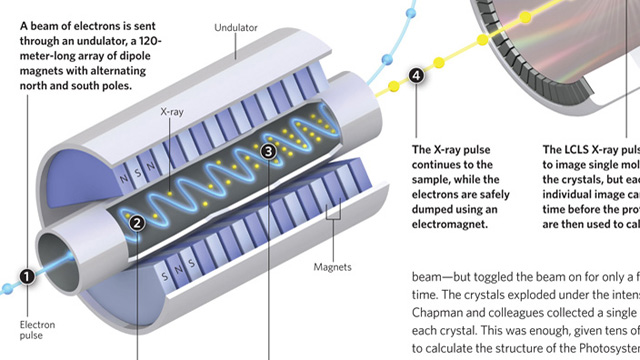
Making high-quality crystals large enough to usefully diffract X-rays is a major headache when attempting to determine protein structures by X-ray crystallography. Researchers prefer crystals that are 100–200 microns in size, with 5 microns being the smallest crystals that can be examined using a synchrotron X-ray source. More powerful X-rays provide better diffraction, but damage the crystals. Henry Chapman, from the Center for Free-Electron Laser Science in Hamburg and colleagues, fed a stream of tiny crystals, as small as 0.2 microns, into an X-ray beam generated by the Linac Coherent Light Source (LCLS)—a billion times more powerful than a synchrotron beam—but toggled the beam on for only a few femtoseconds at a time. The crystals exploded under the intense beam, but not before Chapman and ...



















