
Stay up to date on the latest science with Brush Up Summaries.
What Is the Tumor Microenvironment?
The tumor microenvironment (TME) is a local area created and dominated by a tumor that contains different cell types and chemical signals. It catalyzes tumor growth and can contribute to cancer cells acquiring therapy resistance. Over the last 20 years, the scientific community achieved significant progress in understanding this complex environment. Still, only 3.4 percent of investigated oncology drugs are effective in early trials, which demonstrates how important it is to understand the complex TME and its relation to therapy and resistance.1,2
Key Components of the TME
The TME contains cellular and noncellular components. These include cells of the immune system, cancer associated fibroblasts, and endothelial cells, but also extracellular matrix (ECM), signaling factors, and growth factors (Table 1) that are essential for cancer cell survival.3
Table 1. Cellular and noncellular components of the tumor microenvironment3
| Cell type | Noncellular components |
|---|---|
Immune cells | Extracellular matrix (ECM) |
T-lymphocytes | Exosomes |
B-lymphocytes | Apoptotic bodies |
Macrophages | Chemokines (MIP, CCL11, CCL5, MCP, IL-8, IL-16, CCL9) |
Neutrophils | Cytokines (IL-1, IL-2, IL-3, IL-4, IL-5, IL-6, IL-12, IL-15, IL-18, IL-37, IL-23, IL-27, IL-7, IL-37, IL-31, IL-10) |
Natural killer cells | Growth factors (PDGF, EGF, NGF, TGFα, TGFβ) |
Dendritic cells | Tumor necrosis factor (TNF) |
Other cell types | Interferon gamma (IFN-γ) |
Adipocytes | |
Cancer associated fibroblasts (CAFs) | |
Endothelial cells | |
Cancer cells |
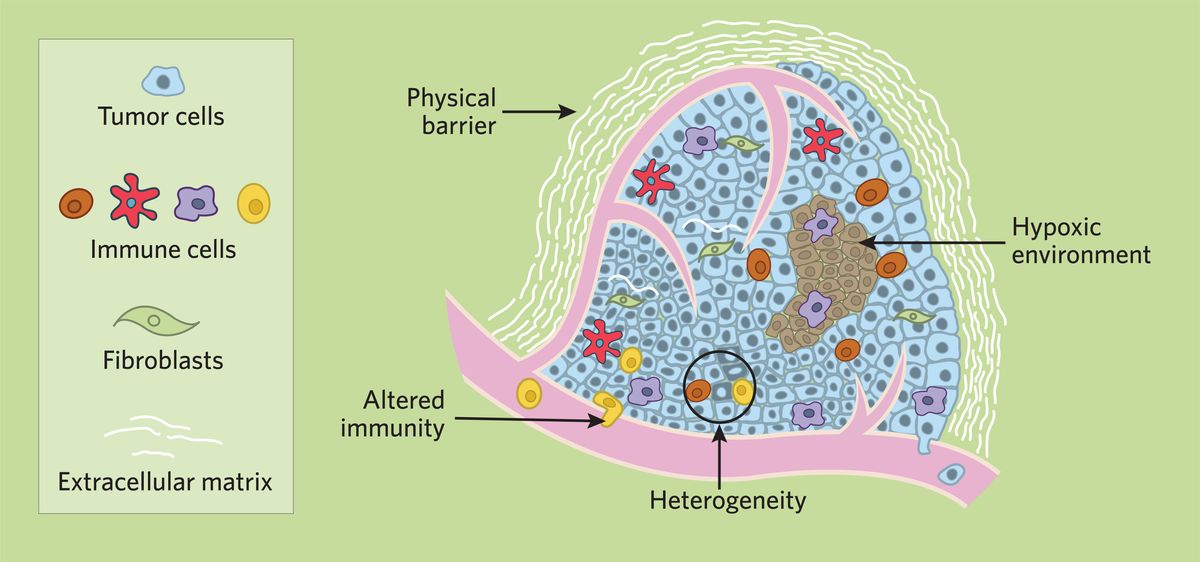
The TME Contributes to Cancer Growth and Survival
Altered immune cells
The TME provides an effective barrier for cancer treatments. It co-opts infiltrating immune cells to promote tumor survival, rather than allowing them to exercise their physiological anti-tumor functions. It achieves this by modifying immune system effectors and reducing immune cell recognition signals. Additionally, the TME reprograms immune cells to release factors that aid cancer cell growth and survival.4
Hypoxic environment
Tumor cells often proliferate faster than new blood vessels can form, so the TME tends to be hypoxic, which recruits immune cells that contribute to ROS production and further increase hypoxia. Hypoxic conditions cause the release of cytokines that enhance cancer cell proliferation, increasing their resistance to apoptosis and altering their metabolism—all key contributors to therapy evasion.5
Physical barrier
The ECM in the TME presents a physical barrier for drug penetrance, significantly reducing the chance to target all tumor cells. Additionally, ECM molecules catalyze the release of transforming growth factor beta (TGF-β), which also promotes therapy resistance in cancer cells.6
Heterogeneity
Lastly, the TME is heterogeneous, and influences cancer cells differently depending on the local area they reside in. This alters gene expression patterns and can cause DNA mutations, contributing to cancer cell instability and heterogeneity, which is a major driver of therapy failure.7
Targeting the TME for Therapy
Researchers have investigated the potential for therapies targeting components of the TME. These strategies include eliminating immune cells that have been co-opted and modified by the tumor, or genetically modifying immune cells to increase their efficiency before administering them into the patient.8 These strategies have given rise to CAR T cell therapies that are successfully in clinical use.9
Antibody therapies
TME and cancer cells express surface antibodies differentially. Using monoclonal antibodies against proteins such as PD-L1, which is highly expressed on cells in the TME, is showing promising results in clinical studies. Approaches like this are often supplemented with radiotherapy or other monoclonal antibodies such as those against Cytotoxic T-Lymphocyte Associated Protein 4 (α-CTL-4), increasing therapy efficiency by reducing the potential of residual tumor cells escaping treatment.10
Perspectives
Researchers currently use cutting edge methodology to further refine their understanding of the TME, identifying weaknesses that allow them to treat the cancer residing within. Such research has the potential to develop prognostic and predictive biomarkers, novel therapies, and informed treatment protocols.
About the Author: Johanna Pruller is a research associate at King’s College London where she previously obtained a PhD in molecular medicine and focused on the development of a gene therapy for alveolar rhabdomyosarcoma. She has since then studied cancer heterogeneity and its consequence on why therapies fail and how tumor cells acquire resistance mechanisms.

- Hartung T. Look back in anger – what clinical studies tell us about preclinical work. ALTEX. 2013;30(3):275-291. doi:10.14573/altex.2013.3.275
2. Mak IW, Evaniew N, Ghert M. Lost in translation: animal models and clinical trials in cancer treatment. Am J Transl Res. 2014;6(2):114-118.
3. Bozyk A, Wojas-Krawczyk K, Krawczyk P, Milanowski J. Tumor microenvironment—a short review of cellular and interaction diversity. Biology. 2022;11(6):929. doi:10.3390/biology11060929
4. Chew V, Toh HC, Abastado JP. Immune microenvironment in tumor progression: characteristics and challenges for therapy. J Oncol. 2012;2012:1-10. doi:10.1155/2012/608406
5. Lluis JM, Buricchi F, Chiarugi P, Morales A, Fernandez-Checa JC. Dual role of mitochondrial reactive oxygen species in hypoxia signaling: activation of nuclear factor-κB via c-SRC– and oxidant-dependent cell death. Cancer Res. 2007;67(15):7368-7377. doi:10.1158/0008-5472.CAN-07-0515
6. Furler R, Nixon D, Brantner C, Popratiloff A, Uittenbogaart C. TGF-β sustains tumor progression through biochemical and mechanical signal transduction. Cancers. 2018;10(6):199. doi:10.3390/cancers10060199
7. Pruller J. Intratumoral heterogeneity as a major challenge for cancer modelling and successful treatment. SciRevsBiology. 2023;2(1). doi:10.57098/SciRevs.Biology.2.1.2
8. Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17(13):4550-4557. doi:10.1158/1078-0432.CCR-11-0116
9. Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3(95). doi:10.1126/scitranslmed.3002842
10. Wang Q, Shao X, Zhang Y, et al. Role of tumor microenvironment in cancer progression and therapeutic strategy. Cancer Med. Published online February 21, 2023:cam4.5698. doi:10.1002/cam4.5698











