In 2018, James Allison and Tasuku Honjo received the Nobel Prize in Physiology or Medicine for their co-discovery of molecular “brakes” that could be released to enhance the immune system’s response against cancer. Their recognition helped immunotherapy gain traction and revolutionized cancer treatment. However, patients’ responses to cancer immunotherapy can vary widely, and many factors influence these—from genetics to the consumption of artificial sweeteners. In this article, learn about some of the factors that can impact cancer immunotherapy responses, as well as how researchers are finding better ways to predict and improve patient outcomes.
Immune Checkpoint Inhibitors Paved the Way for Cancer Immunotherapy
Immune checkpoint inhibitors, popularized by Allison and Honjo’s Nobel Prize, were one of the first cancer immunotherapies that gained FDA approval. In the 1990s, Allison and Honjo identified the negative regulatory roles of two proteins, programmed cell death protein 1 (PD-1) and cytotoxic T lymphocyte-associated protein 4 (CTLA-4), on the immune system. Further research uncovered drugs that block PD-1 and CTLA-4 and, as a result, boost the immune system’s cancer-fighting abilities. This paved the way for the development of other immune checkpoint inhibitors. While PD-1 and CTLA-4 blockers still constitute most of the existing immune checkpoint inhibitors, researchers are also investigating new targets. They are also looking for biomarkers to predict patients’ responses to this class of drugs.
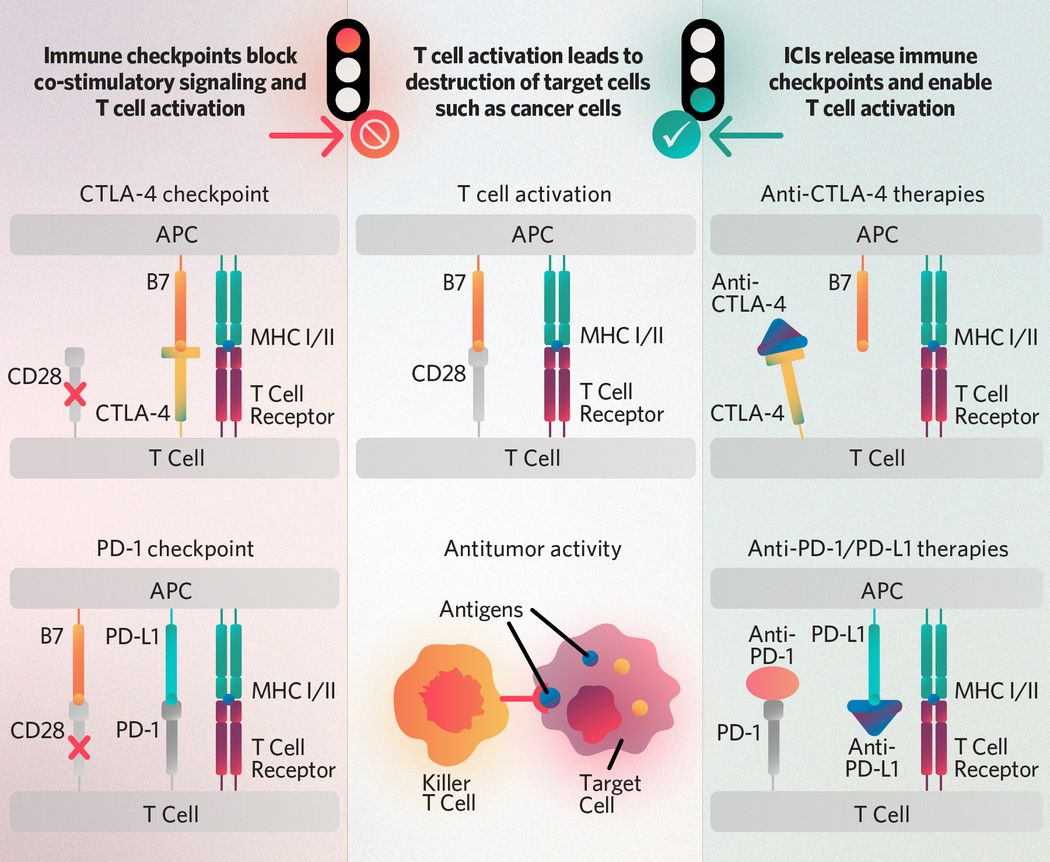
T cell receptors recognize antigens presented on the major histocompatibility complex (MHC I/II) of antigen-presenting cells (APCs) and receive activation signals from co-stimulatory molecule interactions such as those between CD28 and APC-expressed B7-family ligands. The CTLA-4 and PD-1 checkpoints block co-stimulatory activation signaling, and immune checkpoint inhibitors (ICIs) such as anti-CTLA-4, anti-PD-1, and anti-PD-L1 therapies enable it. Once activated, T cells can recognize antigens presented by cancer cells and target tumors for destruction.
The Scientist

Michael Birnbaum, an immunologist at MIT, leads MATCHMAKERS, an international group of scientists who received funding from Cancer Grand Challenges to tackle the problem of how T cells recognize cancer cells.
MIT Biological Engineering
Understanding How T Cells Recognize Cancer Cells May Improve Immunotherapy Outcomes
Scientists know that cancer immunotherapy enhances T cells’ ability to kill tumors, but they still struggle to understand how it does that exactly. T cells use their receptors (TCRs) to recognize their targets, typically in the form of peptides displayed on cell surfaces via major histocompatibility complex (MHC) proteins. But each TCR can recognize thousands of peptide-MHC combinations, and in cancer immunotherapy, hundreds of patients’ T cells are reacting to their tumor. So, how can researchers map specific interactions between TCRs and peptide-MHCs? Michael Birnbaum, an immunologist at the Massachusetts Institute of Technology, is using AI and computational tools to try to close this gap. Birnbaum plans to shed light on T cell antigen recognition using sequencing and structural data. He hopes that this work will not only improve researchers’ understanding of immune responses in cancer but also in other conditions, such as infectious diseases and autoimmunity.

Edison Liu turned to the genetically diverse Collaborative Cross mouse model to answer questions about the role of gene variation in patient responses to cancer immunotherapy.
Edison Liu
A Special Mouse Model Elucidates the Role of Genetics in Variable Cancer Immunotherapy Response
Immunotherapies have revolutionized cancer treatment, but not all patients respond to them. Genetics is a contributing factor, but it’s difficult to figure out to what extent it is responsible, since both the patient’s immune system and the treatment itself likely also produce significant effects. Edison Liu, a cancer biologist at The Jackson Laboratory, compared this to a three-body problem, a physics dilemma stating that it is nearly impossible to determine the exact trajectories of three interacting objects. Recently, Liu’s team used a genetically variable mouse model, called the Collaborative Cross, to study how genetic variability impacts patients’ immunotherapy outcomes. Using these mice, the researchers identified a genetic locus that strongly correlated with positive response to anti-PD-1 treatment. While more work is required to determine how much of the team’s findings are relevant in humans, this advance has brought scientists closer to solving cancer immunotherapy's equivalent of a three-body problem.

Researchers recently found that sucralose could negatively impact cancer patients’ response to immunotherapy and that the gut microbiome likely mediates this relationship.
©iStock, magnez2
Sucralose Consumption Could Weaken Cancer Immunotherapy Response
Perhaps much less expected than genetics, the consumption of non-caloric sweeteners—specifically, sucralose—also seems to affect cancer immunotherapy outcomes. Researchers previously found that artificial sweeteners could alter the gut microbiome and that the gut microbiome could, in turn, influence how well patients respond to cancer immunotherapy. Recently, cancer biologists led by Abigail Overacre-Delgoffe of the University of Pittsburgh discovered that the two phenomena may be linked. The team found that the consumption of sucralose—but not aspartame or saccharin, other commonly used artificial sweeteners—disrupted patients’ responses to cancer immunotherapy. Their follow-up studies in mice suggested that the gut microbiome likely mediated this effect. In the future, Overacre-Delgoffe plans to investigate how artificial sweeteners affect the immune system in other contexts beyond cancer.
AI Could Help Predict Cancer Immunotherapy Outcomes
The first step to improve cancer immunotherapy outcomes is to predict them. Recently, researchers led by Luc Morris, a surgeon and cancer researcher at Memorial Sloan Kettering Cancer Center, and Diego Chowell, a computational immunologist at Icahn School of Medicine at Mount Sinai developed an AI-based tool, called SCORPIO, that uses clinical data to help predict whether patients would respond to immune checkpoint inhibitors. To train SCORPIO, the team used blood test data from about 1,600 patients with different types of cancers who were treated with immune checkpoint inhibitors. The researchers tested SCORPIO's performance using data that it had not seen, during which they found that the tool was significantly better at predicting patient outcomes than existing biomarkers that the FDA has approved for the same purpose. Morris, Chowell, and their colleagues hope that SCORPIO can help physicians determine if immune checkpoint inhibitors are the best treatment option for their patients.
T cells may exhaust during an arduous battle against cancer. Researchers recently developed a tool, called Cyclone, that can monitor the efficacy of drug treatments as they aid T cells in the fight.
©iStock, Peddalanka Ramesh Babu
A Computational Tool Allows Physicians to Monitor T Cell Dynamics After Cancer Immunotherapy
T cell exhaustion, a phenomenon by which T cells gradually lose their killing power, presents another important challenge in cancer immunotherapy. Some researchers, such as Kevin Wang, a medical student at the University of Pennsylvania, think that knowing when T cells get exhausted could tell researchers when patients may need additional treatments, and consequently, improve outcomes. Recently, Wang and his colleagues developed a computational tool, called Cyclone, that could longitudinally monitor immune response weeks after patients underwent immune checkpoint inhibitor treatment. The researchers used Cyclone to track T cell dynamics, such as differentiation state and migration, in stage IV melanoma. They hope to apply the tool to monitor post-immunotherapy response in other types of cancer, such as lung and kidney cancer.