
Delilah Hendriks is a senior postdoctoral researcher at the Hubrecht Institute and Benedetta Artegiani is a group leader at the Princess Máxima Center for Pediatric Oncology (PMC). They co-lead their laboratory at the PMC, where they develop and use human organoids for disease modeling.
The research duo met as postdoctoral researchers in Hans Clevers’s laboratory at the Hubrecht Institute. There, Artegiani and Hendriks stumbled upon a mutation that made liver organoids accumulate fat, mimicking non-alcoholic fatty liver disease (NAFLD), a major cause of chronic liver disease worldwide with currently no effective treatment options.1 NAFLD risk associates with factors such as diets high in fat and sugar, genetic predisposition, and genetic lipid disorders. The disease starts with fat accumulation in the liver, a condition called hepatic steatosis, that can progress to an inflammatory stage that results in liver scarring and failure. While in the Clevers laboratory, Artegiani and Hendriks developed several organoid models of liver disease, which helped provide insights into NAFLD’s progression and treatment options. This work was recently published in Nature Biotechnology.2
Why did you choose to use organoids to study liver disorders and how did you develop liver organoids that model NAFLD?
Benedetta Artegiani: The main reason is because these organoids are human models. We can build models in animals such as mice or zebrafish, but the metabolisms of these animals are very different than humans. We get tissue from human donors and form organoids that can be expanded indefinitely. This provides us with a source of unlimited material to do any type of type of experiment. We started modeling cancer mutations using liver organoids. Then, we came across this super interesting phenotype. When we mutated a gene called APOB, it caused massive fat accumulation in the organoids.
Delilah Hendriks: The fact that the single gene APOB knockout gave us such a strong phenotype was surprising. We wanted to model liver cancer, but for us to find organoids that could grow as a natural fatty liver was quite amazing. Genetics has a huge role in NAFLD. With our organoids, we very precisely introduce these types of mutations and study their phenotypes.
What were the three fatty liver disease models that you produced?
One plus one does not equal two, it equals three because we challenge each other a lot and it is good to see two people’s perspectives.
- Benedetta Artegiani, Princess Máxima Center for Pediatric Oncology
DH: Most people that develop fatty liver disease have a dietary factor. If a person is genetically predisposed to NAFLD but they have a healthy diet, they will not develop the disease. Studies have shown that people with NAFLD have higher levels of free fatty acids compared to healthy people. In our diet model, we provided the organoids with an exogenous source of free fatty acids to mimic the plasma of patients with NAFLD. We saw that if you give a low fatty acid dose, modeling a healthier diet, the organoids got rid of the fat. If we gave them too much, they accumulated fatty acids. Under the microscope, the organoids become dark with visible, round fat droplets.
BA: We also modeled a classic example of NAFLD genetic predisposition caused by the PNPLA3 risk variant. Genetic predisposition means that people do not have a disease upfront, but it can facilitate disease onset in combination with other factors such as diet. We modeled this in two ways—we knocked out the PNPLA3 gene and we used prime editing to introduce a common single nucleotide polymorphism (SNP) in patients that have this genetic predisposition. Organoids in both models had fat accumulation, with fat accumulating in the SNP organoids to a lesser extent. When we used the genetic predisposition organoids in the dietary model, they accumulated more fat than wildtype organoids. This demonstrates a nice synergy between genetics and diet.
DH: To model monogenic lipid disorders, we knocked out either the APOB or the MTTP gene. This gave us our most extreme phenotype—the organoids developed severe and spontaneous steatosis in culture. Patients with monogenic lipid disorders normally develop pediatric fatty liver disease because this is something that does not require a bad diet to develop. These people have a defect where they cannot efficiently export lipids from the cell. We made organoids that accumulated fat at baseline and used them to develop a CRISPR platform that allowed us to see if modifications made that phenotype worse or better.
How did you set up this CRISPR platform and what did you learn?
DH: Having to make organoids fat in a diet model is a complicated system to work with if you want to do a CRISPR screen. Because monogenic lipid disorder organoids are spontaneously fat, we can perform a sequential round of gene editing on them to screen in a high-throughput and genome-wide level the effects of single gene knockouts on the fat phenotype and directly visualize it. We called our CRISPR platform FatTracer because we could literally trace the fat in the microscope. Through that screen, we found a specific and important role for FADS2, a fatty acid desaturase. When we knocked it out, the organoids became full of fat.
BA: We also found that overexpressing FADS2 improved the cell’s fat situation. It was a very striking, black and white phenotype.
Here, we did a smaller, rational screen where we picked genes that might have a role in changing the fat level in the organoids. In the future, one can do a genome-wide screen and test any possible gene to see if it influences or resolves steatosis.
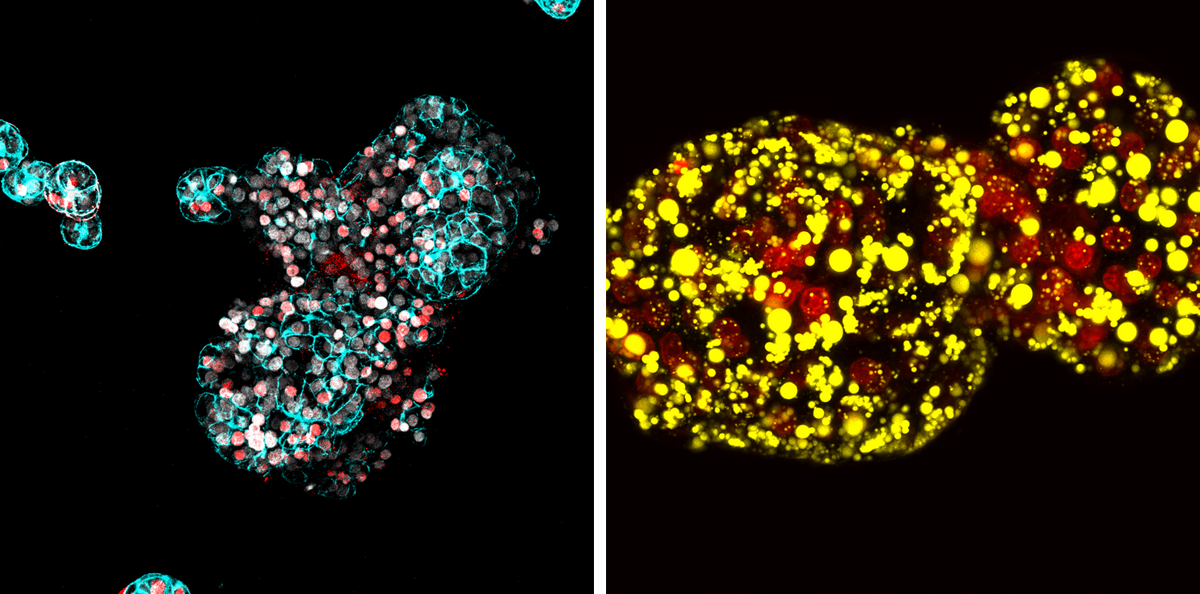
You also performed a drug screen with the fatty liver organoids. What were your most significant findings?
DH: We compiled 17 drugs that have different actions within the liver and tested them in our models to see what they actually do. I was surprised to see that not all drugs that are in development for liver disease are effective at the fatty liver stage. Some drugs may be more effective at combating inflammation, but we wanted to see which actually target the fat. If you do not have fat, you will not have inflammation, and the earlier you can revert or resolve the disease the better. Through this drug screen, we saw that a lot of the effective drugs were the ones that could repress the synthesis of new fatty acids within the cell. This was quite novel. We also studied how the different models responded to drugs, and we saw that their responses were quite different. This means that patients may need different treatments fit to their genetic backgrounds.
BA: We did transcriptomic analyses on the organoids after treating them with drugs that had a positive effect on fat reduction. By clustering the transcriptomes, we predicted the mechanism by which each drug worked. We also predicted adverse drug effects. For example, some drugs resolved the fat phenotype, but they also blocked organoid proliferation, and we could see that from the transcriptome analysis. Before going into clinical trials, this could really be a very powerful platform to evaluate drug effects and possible pitfalls.
What are the benefits of teaming up and forming a joint laboratory group?
BH: We say, one plus one does not equal two, it equals three because we challenge each other a lot and it is good to see two people’s perspectives. Everyone benefits from our strong collaboration, and I think these partnerships are important for science-minded people.
DH: This project was a lot of fun because we could do it together—we could come to each other with our worries and our joys. We always have somebody to share that with. There are more joint labs coming up these days, and it seems to be something that people want because science can be very lonely and challenging at times. Doing it together is great for the science but also great for the people doing the work.
This interview was edited for length and clarity.
- Z.M. Younossi et al., “Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes,” Hepatology, 64:73-84, 2016.
- D. Hendriks et al., “Engineered human hepatocyte organoids enable CRISPR-based target discovery and drug screening for steatosis,” Nat Biotechnol, 1-15, 2023.














