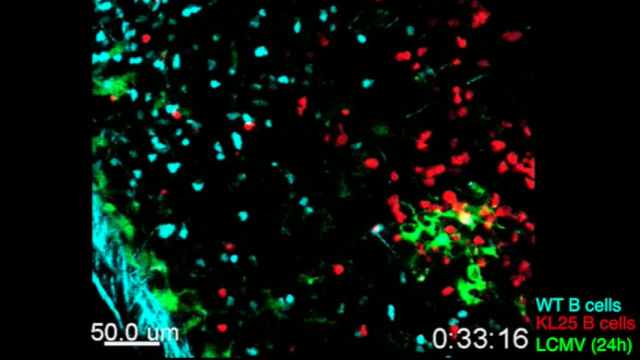
 LCMV-specific B cells (red) show restricted movement, directionality, and proliferation during chronic LCMV infection.SCIENCE IMMUNOLOGY, S. SAMMICHELI ET AL.Most viral infections trigger B cells to produce neutralizing antibodies, but for a handful of viruses that cause chronic infections, B cells are for some reason unable to do their job. The biggest driver for this suppression, at least for the mouse lymphocytic choriomeningitis virus (LCMV), turns out to be a group of cytokines collectively called type I interferon (IFN-I), according to three independent reports published today (October 21) in Science Immunology.
LCMV-specific B cells (red) show restricted movement, directionality, and proliferation during chronic LCMV infection.SCIENCE IMMUNOLOGY, S. SAMMICHELI ET AL.Most viral infections trigger B cells to produce neutralizing antibodies, but for a handful of viruses that cause chronic infections, B cells are for some reason unable to do their job. The biggest driver for this suppression, at least for the mouse lymphocytic choriomeningitis virus (LCMV), turns out to be a group of cytokines collectively called type I interferon (IFN-I), according to three independent reports published today (October 21) in Science Immunology.
“All of the three papers identified type I IFN as the culprit,” said Matteo Iannacone, an immunologist at the San Raffaele Scientific Institute in Milan who coauthored one of the studies.
For decades, immunologists have used LCMV as a model system to study T cell–dominant immune responses because B cell production of virus-neutralizing antibodies is absent or weak and delayed for several weeks. But because some human viruses, such as HIV and hepatitis B (HBV), also fail to induce strong antibody responses, Dorian McGavern, a viral immunologist at the National Institute of Neurological Disorders and Stroke, and others decided to use LCMV to find mechanisms behind B-cell failure.
McGavern’s team started by injecting uninfected mice with B cells engineered to recognize LCMV, then exposed them ...


















