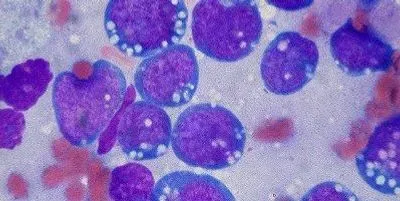
Burkitt’s lymphomaFLICKR, ED ETHMANActivation-induced cytosine deaminase (AID) is an enzyme critical for protecting against malaria and other infectious diseases. It may also play a key role in triggering Burkitt’s lymphoma, the most common childhood cancer in sub-Saharan Africa, according to a study published today (August 13) in Cell. Although scientists have noted a connection between malaria and Burkitt’s lymphoma for decades, uncovering the biological mechanism behind the association proved elusive. Scientists at Rockefeller University in New York City have now shown that prolonged malaria infection in mice boosts B-lymphocyte and AID production, and that elevated AID likely leads to Burkitt’s lymphoma.
Burkitt’s lymphomaFLICKR, ED ETHMANActivation-induced cytosine deaminase (AID) is an enzyme critical for protecting against malaria and other infectious diseases. It may also play a key role in triggering Burkitt’s lymphoma, the most common childhood cancer in sub-Saharan Africa, according to a study published today (August 13) in Cell. Although scientists have noted a connection between malaria and Burkitt’s lymphoma for decades, uncovering the biological mechanism behind the association proved elusive. Scientists at Rockefeller University in New York City have now shown that prolonged malaria infection in mice boosts B-lymphocyte and AID production, and that elevated AID likely leads to Burkitt’s lymphoma.
“It’s really groundbreaking for moving the field forward,” said Rosemary Rochford, an immunologist and microbiologist at the University of Colorado, Denver, who was not involved in the research. “This has been a key question in the field for a long time: What’s the role of malaria in promoting Burkitt’s lymphoma?”
Although there was ample epidemiological evidence associating malaria infection with Burkitt’s lymphoma, one challenge was the lack of an animal model to investigate the connection. Rochford noted that the transgenic mice used in the present study fill that gap. “It’s the first animal model that’s been found to demonstrate that Plasmodium infection can promote the development of lymphoma,” she said.
Rockefeller’s Davide Robbiani, Michel Nussenzweig and colleagues infected wild-type mice with Plasmodium chabaudi, causing long-term ...