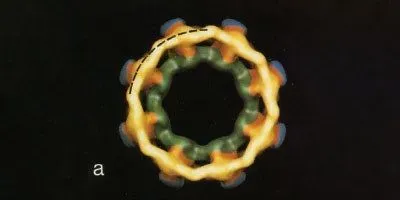
STATE OF THE ART: Using a novel combination of imaging techniques, Ronald Milligan, a cell biologist at the Scripps Research Institute, and two of his colleagues published this model of a Xenopus laevis nuclear pore in 1992. Rather than examining one electron micrograph at a time, the researchers averaged many images together for more-reliable results. And by tilting their samples at various angles, they were able to translate flat images into a three-dimensional model, revealing an unexpected dimension of symmetry.CELL, 69:1133-41, 1992, COURTESY RON MILLIGANWhen he was a postdoc in the 1970s, Ronald Milligan came across a grainy electron micrograph in a textbook that depicted the nuclear pore complex, a relatively unstudied but crucial structure responsible for shuttling molecules in and out of the nucleus. The blurry image, published in 1974 by Alexander Fabergé of the University of Texas at Austin, showed something remarkable: the pores seemed fashioned out of eight identical parts. “I remember taking it back to my advisor and saying, ‘Look at this!’” he recalls, and they launched their pursuit of a better image.
STATE OF THE ART: Using a novel combination of imaging techniques, Ronald Milligan, a cell biologist at the Scripps Research Institute, and two of his colleagues published this model of a Xenopus laevis nuclear pore in 1992. Rather than examining one electron micrograph at a time, the researchers averaged many images together for more-reliable results. And by tilting their samples at various angles, they were able to translate flat images into a three-dimensional model, revealing an unexpected dimension of symmetry.CELL, 69:1133-41, 1992, COURTESY RON MILLIGANWhen he was a postdoc in the 1970s, Ronald Milligan came across a grainy electron micrograph in a textbook that depicted the nuclear pore complex, a relatively unstudied but crucial structure responsible for shuttling molecules in and out of the nucleus. The blurry image, published in 1974 by Alexander Fabergé of the University of Texas at Austin, showed something remarkable: the pores seemed fashioned out of eight identical parts. “I remember taking it back to my advisor and saying, ‘Look at this!’” he recalls, and they launched their pursuit of a better image.
Milligan was taken in by indications that this tiny gateway had a symmetrical design. From sea anemones to flower petals, symmetry is everywhere in nature, and scientists were only just beginning to open a window onto the molecular world, revealing its varied architecture through advances in microscopy. Even those early images of the nuclear pore “are so dramatic,” Milligan says. “Everyone is drawn to them. They’re like little flowers.”
Images produced by Milligan and others over the next decade revealed only a fuzzy ring, and “very few of them gave these hints of eightfold symmetry.” It was not until 1992 that Milligan finally resolved the basic shape and structure of this mysterious portal in the nuclear membrane. Milligan had just established a molecular microscopy lab ...