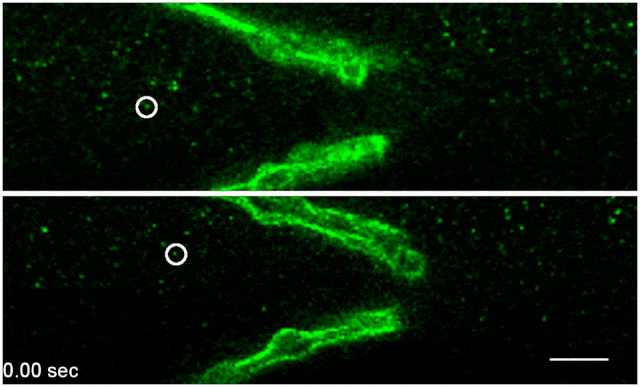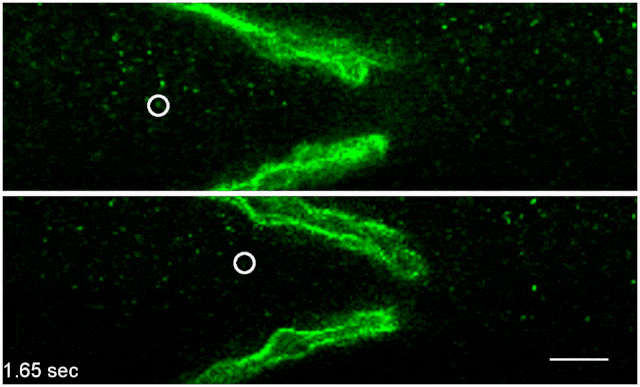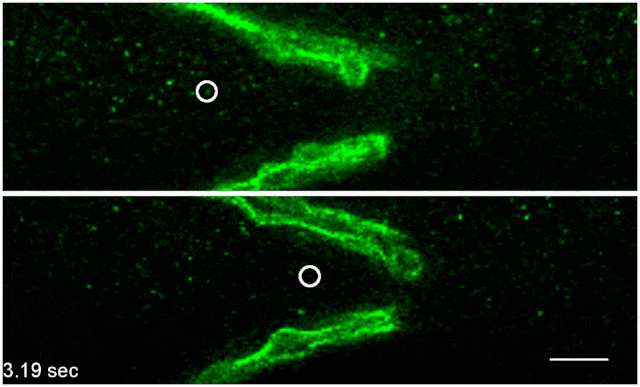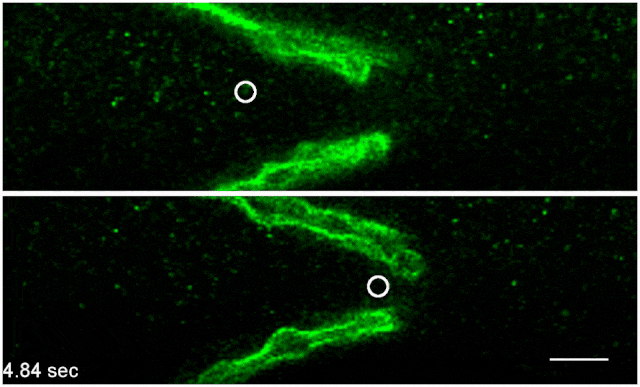
 Green fluorescently-tagged nanospheres flow through a lymph vessel from an unstressed mouse (top) and a mouse that has been administered the stress hormone norepinephrine (bottom). Scale bar: 20 μmNATURE COMMUNICATIONS, LE ET AL.
Green fluorescently-tagged nanospheres flow through a lymph vessel from an unstressed mouse (top) and a mouse that has been administered the stress hormone norepinephrine (bottom). Scale bar: 20 μmNATURE COMMUNICATIONS, LE ET AL.
Stress is implicated in increased tumor progression risk and poor survival in cancer patients. A number of recent studies have linked these effects to the promotion of tumor cell dissemination through the bloodstream via stress-induced pathways. Now, a mouse study led by researchers in Australia has revealed the mechanisms by which stress modulates cancer’s spread through another transport network open to tumor cells—the lymphatic system. The findings were published today (March 1) in Nature Communications.
“Stress not only affects your well-being, but it also affects your biology,” said study coauthor Erica Sloan, a cancer researcher at Monash University in Melbourne. “Our study particularly highlights the early steps of tumor cell dissemination into the lymphatic system.”
“This is an excellent contribution,” said Kari Alitalo, a professor ...




















