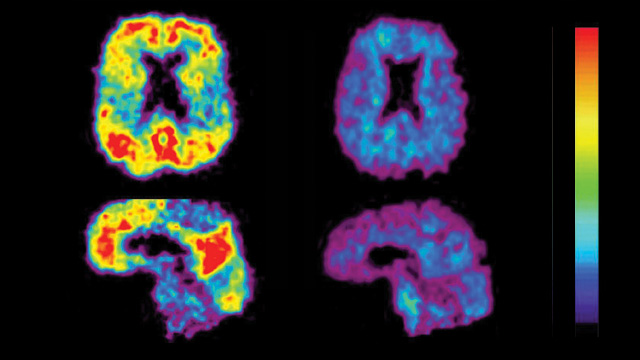
 READY FOR USE IN CLINICAL TRIALS: Comparative PET scan views of a patient with Alzheimer’s disease (left) and an elderly person with no cognitive impairment (right). The red and yellow areas show high concentrations of fluorescent dyes developed to detect amyloid plaques. UNIVERSITY OF PITTSBURGH, PET AMYLOID IMAGING GROUP
READY FOR USE IN CLINICAL TRIALS: Comparative PET scan views of a patient with Alzheimer’s disease (left) and an elderly person with no cognitive impairment (right). The red and yellow areas show high concentrations of fluorescent dyes developed to detect amyloid plaques. UNIVERSITY OF PITTSBURGH, PET AMYLOID IMAGING GROUP
There’s been a lot of discussion about the need for biomarkers to diagnose and monitor the progression of Alzheimer’s disease (AD). That discussion should continue, for sure, but the aging of our society means that researchers in the AD field need to move from talk to action.
Although some biomarkers have been under study for more than a decade, the continuing absence of truly effective therapies for preventing, moderating, or curing AD spurred the publication in December of a special supplemental issue of Neurobiology of Aging about the latest research and expert opinion on AD biomarkers in an effort to advance trials of experimental agents for the disease.
Biomarkers for AD can be divided broadly into those that identify aspects of the underlying molecular pathology ...




















