Lipid nanoparticles (LNPs) have emerged as pivotal tools for delivering modern therapeutics from mRNA vaccines to gene-editing platforms. These nanoscale capsules protect fragile cargo and target specific cells, but understanding their structure and function remains challenging. Traditional characterization methods fall short, averaging out key variables across heterogeneous populations. Now, super-resolution imaging is offering an unprecedented look at LNPs on the individual particle level, enabling researchers to visualize and quantify features such as size, ligand density, and cargo encapsulation in real time.

Ricardo Nunes Bastos, PhD Vice President of Strategy Oxford Nanoimaging (ONI)
In this Innovation Spotlight, Ricardo Nunes Bastos, vice president of strategy at Oxford Nanoimaging (ONI), explains important factors when working with LNPs and highlights how the leap in imaging resolution and throughput is reshaping how scientists develop and refine nanoparticle-based treatments.
What are LNPs and how are they used in therapeutic development?
One should think of LNPs as lab-engineered spheres, about the size of a virus, that act as high-tech delivery vehicles for therapeutic agents. These miniature capsules are revolutionizing how we deliver treatments across the body. Their superpower is protecting delicate cargo, such as drugs or mRNA, from being broken down before reaching their destination. Once the LNPs encounter their target cells, they can merge directly with cell membranes, like little molecular couriers slipping past security. This fusion allows them to release their payload exactly where it’s needed, enhancing the treatment’s effectiveness while minimizing unwanted side effects. I think today everybody probably knows about LNPs because they really stepped into the spotlight with the COVID-19 mRNA vaccines. But their story began back in 2018 when the first LNP-based therapy was approved. Since then, they’ve rapidly expanded their role in medicine, becoming essential for everything from mRNA vaccines to cutting-edge gene therapies.
What are some important considerations for researchers producing LNPs?
Producing effective LNPs is both an art and a science. First off, researchers need to nail down how to load their precious cargo and purify the particles without compromising their integrity. But that’s just the beginning of their journey from the lab bench to the patient. That means efficacy, scalability, and especially stability during storage and transportation are essential factors to keep in mind. It’s not enough to have a breakthrough formulation in the lab; it has to hold up in the real world. One additional tricky issue is making sure LNPs deliver their cargo to the right cells or tissues. Most LNPs naturally gravitate toward the liver, which is helpful in some contexts but a hurdle in others. That’s where ligands come in. These are surface molecules that help stabilize the particles and guide them to specific destinations. But fine-tuning this targeting is difficult and not always predictable.
What are the main challenges in LNP production and characterization?
One of the biggest hurdles is manufacturing particles that meet all the critical specs—not just in yield, but in terms of size, structural stability, how much cargo they carry, and how well their ligands are functionalized. You want each particle to do its job, and you want that performance to be consistent from batch to batch, in the hospital next to you and on the other side of the world.
Because LNPs as I mentioned are tiny, between 50 and 200 nanometers, controlling and accessing key characteristics such as morphology, cargo loading, or ligand distribution is extremely challenging. Most bulk characterization methods assume that all particles in a sample are the same, which just isn’t true. Even small variations can have a big impact on therapeutic outcomes. Many current methods lack the sensitivity or resolution to evaluate individual LNPs. You might get data on average size or charge, but you won’t know how many particles are actually loaded with cargo or whether ligand attachment is uniform.
Cryogenic electron microscopy has been helping and gives beautiful, high-resolution images, but it’s slow, labor-intensive, and low throughput. That’s a huge limitation when one is trying to understand or optimize a complex, scaled-up manufacturing process. The field is moving toward next-generation tools that offer single-particle resolution and can correlate multiple properties, such as size, cargo load, and ligand density, in real time. That kind of multi-parametric analysis is what’s going to unlock the next wave of precision therapeutics.
How can super-resolution imaging help circumvent these challenges?
Traditional fluorescence microscopy is limited by the diffraction limit of light, which means one can't resolve anything smaller than 200 nanometers. LNPs often fall below that threshold, so conventional tools just don’t cut it. Super-resolution imaging breaks that barrier, allowing researchers to see and quantify individual LNPs, even when they’re well below 200 nm in size down to 20 nm. That’s a big deal. Now, instead of working with averages and assumptions, we can directly observe particle size, shape, ligand loading, and cargo encapsulation—not just in bulk, but for every single nanoparticle. It opens a lot of possibilities. For one, you can now image LNPs in two different contexts; immobilized on a glass surface for precise structural analysis, or inside living cells to observe their behavior in real time. Some super-resolution techniques even support live-cell assays, so you can study how LNPs are taken up, where they travel inside the cell, and how efficiently they release their cargo.
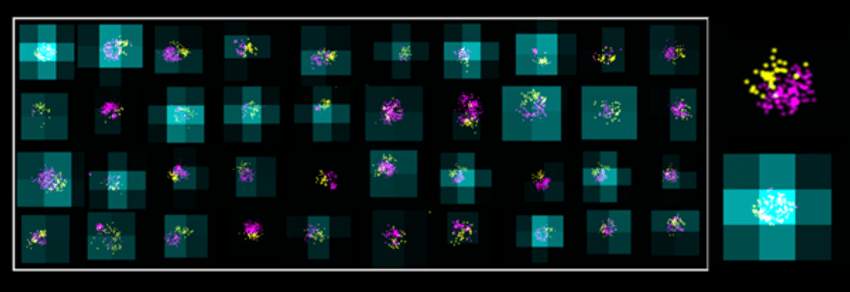
Super-resolution imaging of lipid nanoparticles can show their size (magenta), surface engineering (yellow), and mRNA encapsulation (cyan), as shown in this mosaic view of different LNPs from the same formulation.
Oxford Nanoimaging (ONI)
Super-resolution imaging has clear advantages in throughput and information richness. Cryogenic electron microscopy is fantastic for ultrastructure, but you’re typically looking at just 500 particles per sample. Super-resolution methods can analyze thousands of particles, capturing multiple metrics in a single assay.
Compare that to methods such as dynamic light scattering or Ribogreen quantification, which require parallel experiments just to correlate population-level data. Super-resolution imaging combines all that in one workflow, making it faster, more precise, and much more insightful. In my opinion, this is transformative. With super-resolution, researchers can optimize LNP formulations with unprecedented precision. You’re not guessing, you’re measuring.
Ultimately, this technology is accelerating the development of RNA-based drugs, mRNA vaccines, and gene-editing platforms. It gives scientists a real-time window into nanoscale biology—something we could only dream of a decade ago.
How can scientists use super-resolution imaging to characterize LNPs in their laboratories?
Thanks to advances like ONI’s LNP Profiler workflow, super-resolution imaging is no longer reserved for elite imaging facilities. The LNP Profiler is a complete, end-to-end platform designed to make nanoscale characterization of LNPs accessible to any laboratory. It allows researchers to capture, visualize, and quantify immobilized LNPs with nanometer precision, delivering incredibly detailed insights on particle size, cargo and ligand distribution, structural integrity, and overall composition. The key metric we focus on is the so-called loaded fraction—a measurement of how many particles are actually carrying their intended cargo.
ONI’s LNP Profiler workflow is designed for full automation and accessibility. It starts with a novel functionalized surface that’s optimized for capturing PEGylated LNPs, which are widely used due to their enhanced stability and low immunogenicity, without interfering with downstream measurements. We’ve also tackled one of the biggest pain points in single-molecule localization microscopy—the need for finicky imaging buffers. With the LNP Profiler, we sidestep that entirely by using a new class of self-blinking fluorophores. These allow for high-resolution imaging without compromising particle integrity, even when measuring sensitive cargos such as mRNA.
But perhaps the most groundbreaking work came in the form of machine learning (ML) integration. To classify whether an LNP contains cargo, we developed a classifier-based ML model that reliably determines cargo positivity. And to make sense of heterogeneous populations, we implemented ML clustering algorithms that group particles based on shared traits—without needing manual parameter tuning. It is incredibly powerful and requires minimal user input.
I know a common concern about super-resolution imaging is that it’s seen as a low-throughput technique. We’ve completely flipped the script on this. Our AutoLNP platform can characterize over 10,000 LNPs per sample—and not just for one metric, but for all of them simultaneously: size, shape, cargo, ligand, and integrity. Users can run 16 or more samples in a single day, with results and insights ready in under 8 hours. We are also taking automation to the next level: Our new Aplo sample prep is nearly 100 percent hands-off and imaging, optimization, and reporting are fully automated. This means that even researchers without any prior experience with super-resolution imaging can get up and running with little training.
What excites you the most about the future of LNP development and characterization using super-resolution imaging?
We are standing at the edge of a revolution in nanomedicine, and super-resolution imaging is at the heart of it. What excites me most is how this technology is enabling us to dive into LNPs with unprecedented precision just as the field is exploding into personalized medicine, gene therapy, and beyond. We're no longer talking about niche research—we're talking about real-world scalability, cost-effectiveness, and therapeutic impact. The technology is mature, the tools are ready, and the next generation of LNP-based therapies is coming fast. With super-resolution, we can now analyze those bespoke formulations particle by particle, revealing critical insights that bulk techniques simply miss. We’re making it possible for any laboratory, academic, clinical, or commercial, to understand LNP behavior at the single-particle level. This means faster optimization of formulations, better therapeutic performance, and a deeper understanding of how these particles function biologically. In short, super-resolution imaging is no longer just a fancy microscope—it’s a vital part of the LNP research toolkit, unlocking answers we couldn’t access before. That’s what makes this moment so exciting.





















