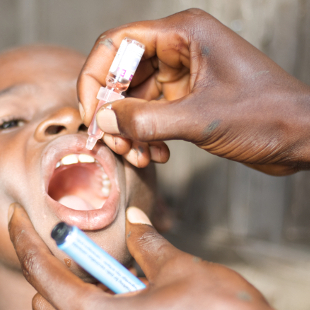
 FLICKR, JULIEN HARNEISThe international spread of polio from Pakistan, Syria, and Cameroon to Afghanistan, Iraq, and Equatorial Guinea, respectively, which last month caused World Health Organization (WHO) officials to declare a public health emergency, serves as a devastating reminder that, although vaccination campaigns have squelched polio in most of the world, eradication is not complete. As of June 11, the virus has sickened 79 people in those three polio-exporting countries and 94 individuals worldwide this year alone. That’s up from 55 cases worldwide through June last year.
FLICKR, JULIEN HARNEISThe international spread of polio from Pakistan, Syria, and Cameroon to Afghanistan, Iraq, and Equatorial Guinea, respectively, which last month caused World Health Organization (WHO) officials to declare a public health emergency, serves as a devastating reminder that, although vaccination campaigns have squelched polio in most of the world, eradication is not complete. As of June 11, the virus has sickened 79 people in those three polio-exporting countries and 94 individuals worldwide this year alone. That’s up from 55 cases worldwide through June last year.
Vaccination campaigns and global public health initiatives have caused worldwide cases of polio infection to drop from more than 350,000 reported in 1988, giving researchers reason to hope the infectious disease was near defeat. But as the WHO points out on its website, “as long as a single child remains infected with poliovirus, children in all countries are at risk of contracting the disease.”
In addition to the formidable challenge of vaccinating every child to halt disease transmission, another problem threatens to keep complete eradication out of reach. After immunization with the live oral polio vaccine, which is used in most of the developing world, children with impaired antibody production fail to clear the infection. These immune-deficient patients can continue to excrete poliovirus in their ...






















