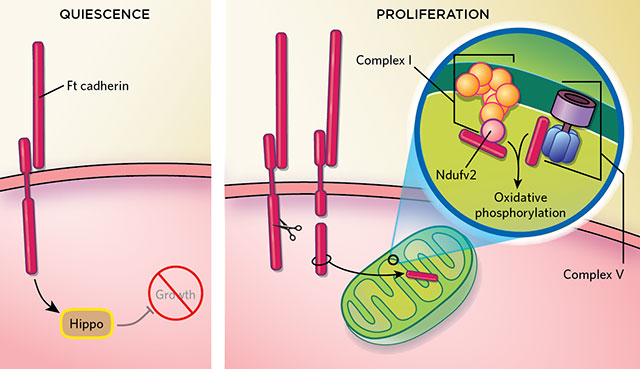
 ENERGY SWITCH: In quiescent fruitfly cells, a cell-adhesion molecule, Ft cadherin, inhibits growth by activating the Hippo pathway (above, left). Upon the severing of the cytosolic arm of Ft cadherin, the liberated piece enters a mitochondrion, where it interacts with two groups of proteins, Complex I and Complex V, promoting oxidative phosphorylation and lifting Hippo’s repression of growth (above, right). © KIMBERLY BATTISTA, BASED ON A. SING ET AL., CELL, 158:1293-1308, 2014.
ENERGY SWITCH: In quiescent fruitfly cells, a cell-adhesion molecule, Ft cadherin, inhibits growth by activating the Hippo pathway (above, left). Upon the severing of the cytosolic arm of Ft cadherin, the liberated piece enters a mitochondrion, where it interacts with two groups of proteins, Complex I and Complex V, promoting oxidative phosphorylation and lifting Hippo’s repression of growth (above, right). © KIMBERLY BATTISTA, BASED ON A. SING ET AL., CELL, 158:1293-1308, 2014.
The paper
A. Sing et al., “The atypical cadherin Fat directly regulates mitochondrial function and metabolic state,” Cell, 158:1293-1308, 2014.
When clustered together in tissues, cells sense and respond to their neighbors by way of membrane receptors, which relay signals informing individual cells when and in what directions to multiply. The loss of—or failure to respond to—these cues can spill over to cause cancer or other diseases. Researchers previously knew that one such surface receptor, a conserved cell-adhesion molecule dubbed Fat (Ft) cadherin, functioned in two ways, regulating tissue organization and a tumor-suppressive signaling pathway known as Hippo. Now, a new study suggests this molecule may have a third role: linking cell proliferation to mitochondrial respiration.
In 2003, Helen McNeill of the University of Toronto ...



















