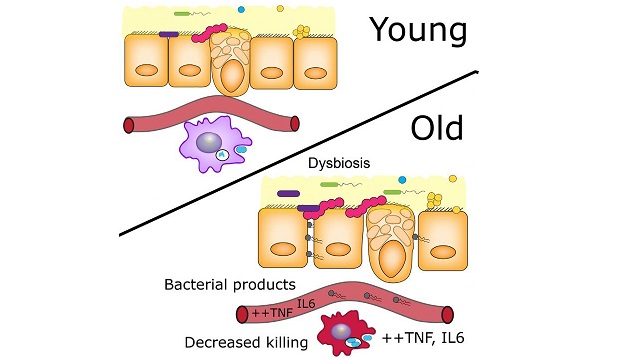
 Using young and old germ-free and conventional mice, the authors demonstrated that age-related microbiota changes drive intestinal permeability, age-associated inflammation, and decreased macrophage function. Reducing TNF levels rescued microbiota changes and protected old mice from intestinal permeability, the researchers reported.CELL HOST & MICROBE, N. THEVARANJAN ET AL.Constant, low-level inflammation, increases as animals age. Until now, it wasn’t clear why. In a study published today (April 12) in Cell Host & Microbe, researchers have described a causal connection between aging-related changes in the gut microbiota and inflammation levels in mice.
Using young and old germ-free and conventional mice, the authors demonstrated that age-related microbiota changes drive intestinal permeability, age-associated inflammation, and decreased macrophage function. Reducing TNF levels rescued microbiota changes and protected old mice from intestinal permeability, the researchers reported.CELL HOST & MICROBE, N. THEVARANJAN ET AL.Constant, low-level inflammation, increases as animals age. Until now, it wasn’t clear why. In a study published today (April 12) in Cell Host & Microbe, researchers have described a causal connection between aging-related changes in the gut microbiota and inflammation levels in mice.
The study “helps us better understand the cause and effect relationships between age-related changes in the microbiota and the health of the aging host organism,” said David Walker of the University of California, Los Angeles, who did not participate in the work. “It’s well established that as all organisms age, there’s an increase in inflammation, but the underlying cause of that increase is poorly understood. This work is quite clear in showing that the microbiota itself is actually playing a causal role in these age-related increases in inflammation.”
“If you have higher-than-average inflammation in your blood, then you are more likely to die early, to be less healthy, to be less active, to be more frail, and to have chronic inflammatory diseases,” coauthor Dawn Bowdish ...





















