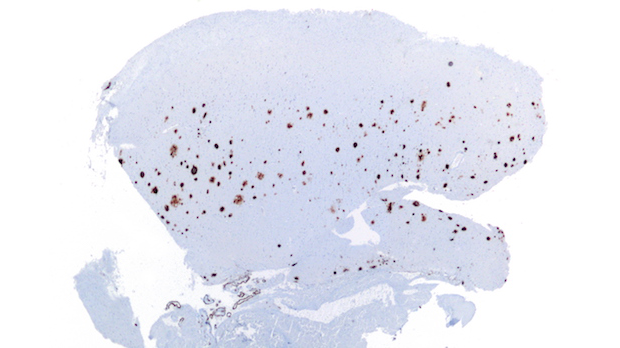
 Amyloid-β plaques in a brain histological sample WIKIMEDIA, NEPHRONAmyloid-β proteins form plaques in the brain that are a hallmark of Alzheimer’s disease. Designed to clear these plaques, the antibody aducanumab is demonstrating early success after a year of testing in a Phase 1b clinical trial, according to interim results published today (August 31) in Nature. The results also provide tantalizing—but tenuous—evidence to suggest that the therapy helps prevent Alzheimer’s disease–associated cognitive decline in a dose-dependent fashion.
Amyloid-β plaques in a brain histological sample WIKIMEDIA, NEPHRONAmyloid-β proteins form plaques in the brain that are a hallmark of Alzheimer’s disease. Designed to clear these plaques, the antibody aducanumab is demonstrating early success after a year of testing in a Phase 1b clinical trial, according to interim results published today (August 31) in Nature. The results also provide tantalizing—but tenuous—evidence to suggest that the therapy helps prevent Alzheimer’s disease–associated cognitive decline in a dose-dependent fashion.
“What they found was very exciting: that aducanumab treatment was associated with an unusually striking and progressive removal of existing plaques,” said Eric Reiman, executive director of the Banner Alzheimer’s Institute in Phoenix, Arizona, who wrote an accompanying editorial but was not involved in the work.
Previous immunotherapies have shown relatively modest plaque-clearing—“maybe reversing a little bit of amyloid deposition,” Reimer told The Scientist. “But this is the most striking effect so far.”
Aducanumab, developed by Cambridge, Massachusetts–based Biogen, was discovered in a screen of human memory B cells that produce antibodies capable of binding amyloid-β.
The primary goal of this Phase 1b trial was to determine a safe dose at which antibodies might help clear amyloid-β plaques. The researchers enrolled 165 study participants, 50 ...





















