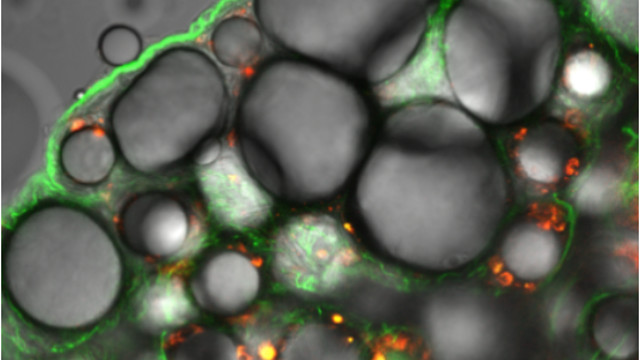
 Adipocytes and vascular endothelial cells expressing red fluorescent protein delivered by an adipocyte targeting peptide (green).YONG-HEE KIM
Adipocytes and vascular endothelial cells expressing red fluorescent protein delivered by an adipocyte targeting peptide (green).YONG-HEE KIM
The technique: A specific short peptide aimed at fat cells can deliver a DNA sequence that knocks down expression of a key fatty acid binding protein. Mice on a high-fat diet treated with this molecular complex showed reduced body weight and improved metabolic profiles. The method, reported today (October 5) in Nature Materials, provides a new way to study the functions of adipocytes—a notoriously intractable cell type—and suggests an avenue for gene therapy to combat obesity in humans.
“It’s always welcome when there is a new technology to deliver specific targeting molecules, whether they’re RNAi or small molecules, to restricted parts of the body,” said Gökhan Hotamisligil, a professor at the Harvard School of Public Health who was not involved in the study. Hotamisligil, who ...




















