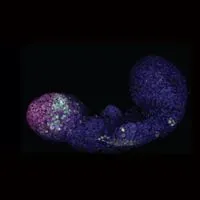
ABOVE: A five-day-old gastruloid
DAVID TURNER
Clumps of embryonic stem cells given one brief molecular stimulation start arranging themselves in a way that resembles a mouse embryo, down to the timing of when the cells switch on particular developmental genes, according to a report in Nature today (October 3). The reproducible formation of these so-called gastruloids could make them a potentially valuable resource for research and reduce the number of animals used in experimentation.
“This is a fascinating study,” developmental biologist James Briscoe of the Frances Crick Institute in the UK writes in an email to The Scientist. “The ability of ES [embryonic stem] cells, when properly shepherded, to specify all three major body axes is striking and is yet another example of the surprising ability of developing tissues to self-organize.”
ES cells are derived from a group of cells within the early mammalian embryo called the inner cell mass. During ...