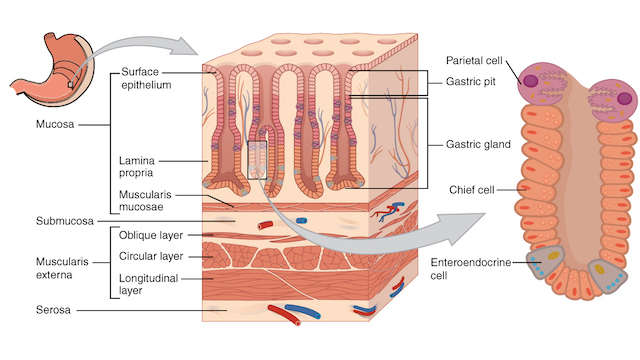
 WIKIMEDIA, OPENSTAX COLLEGEIn healthy individuals, chief cells, which are found at the base of corpus glands in the stomach, act as producers of digestive enzymes. However, if the gut undergoes damage or genetic mutation, these cells have the ability to convert into stem cells that can lead to gastric cancer, according to a study published last week (June 5) in Nature Cell Biology.
WIKIMEDIA, OPENSTAX COLLEGEIn healthy individuals, chief cells, which are found at the base of corpus glands in the stomach, act as producers of digestive enzymes. However, if the gut undergoes damage or genetic mutation, these cells have the ability to convert into stem cells that can lead to gastric cancer, according to a study published last week (June 5) in Nature Cell Biology.
“This is the first definitive demonstration that that a subset of chief cells is cancer prone and can serve as the origin of gastric cancer,” says study coauthor Nick Barker, who studies cancer and stem cells at A*STAR Institute of Medical Biology (IMB) in Singapore.
Still, the identity of gastric-cancer forming cells has been controversial, and not all researchers are convinced Barker’s study has resolved the debate.
A long-standing theory in gastroenterology is that stem cells in the stomach arise in the isthmus, the region located around the middle of the corpus glands. These cells migrate to the tops and bottoms of the glands, replenishing lost cells and maintaining homeostasis. Mutations in these stem cells, many scientists believe, can lead to precancerous lesions, such as ...























