There is no single genetic blueprint for cancer. Instead, each individual cancer draws on a collection of acquired mutations that endow the cells with a selective advantage and superior immune evasion and proliferation tactics. Thanks to next-generation sequencing technologies, many patients diagnosed with a particular cancer can discover whether their tumors harbor specific mutations that render them more susceptible to particular therapies. However, targeted approaches fail to capture the full suite of alterations and biomarkers nestled in the complex genetic architecture of a patient's tumor, potentially obscuring the best available treatment plan for an individual patient.
In a study published in Nature Medicine, researchers developed a bioinformatics pipeline for integrating whole-genome sequencing (WGS) data from 13,880 tumors with matching patient clinical data.1 The large-scale study revealed somatic and germline DNA mutations that affect prognosis, highlighting the potential influence of comprehensive cancer genomics on patient outcomes.

“Cancer is a disease of disordered genomes,” said Mark Caulfield, a genomic medicine researcher at Queen Mary University of London and a coauthor of the study. Many of the genetic mishaps that emerge are irrelevant to the cancer’s growth, but others carry information that would allow researchers to predict the tumor’s progression and vulnerabilities. Caulfield and his team set out to find these information-rich genetic fingerprints.
For many cancers, genomic testing to identify specific gene variants, like single-nucleotide polymorphisms, is already part of standard care. However, these targeted panels are limited in scope. “[A comprehensive approach] gives you the full picture of what’s happening in an individual patient’s tumor that you might miss if you just end up using targeted panels,” said Arul Chinnaiyan, a cancer researcher at the University of Michigan who was not involved in the study. “This study really brings together the importance of that.”
To explore the genes that shape health and disease, the UK government established the 100,000 Genomes Project in 2013.2 Run by the government-owned company Genomics England, researchers set out to sequence the genomes of patients within the National Health Service (NHS), the UK’s publicly funded healthcare system. Within the Cancer Program of the initiative, researchers and clinicians teamed up to identify the genetic changes that drive cancer progression. In the present study, the researchers sequenced the patients’ tumors alongside their matched germline DNA. Analyzing a patient's normal, inherited DNA alongside tumor DNA can provide a bigger picture of susceptibility to other cancers or inform clinicians on which treatments are more appropriate for the patient given any genetic variants that affect drug metabolism.3
The 13,880 samples covered 33 tumor types. In a single test, WGS can detect somatic small variants, including single nucleotide variants (SNV) and insertions and deletions, and copy number aberrations (CNA). By integrating longitudinal, real-world clinical data, the researchers could assess the role of these genomic factors in treatment response and survival.
“The entire scope of the study was quite impressive,” said Chinnaiyan.
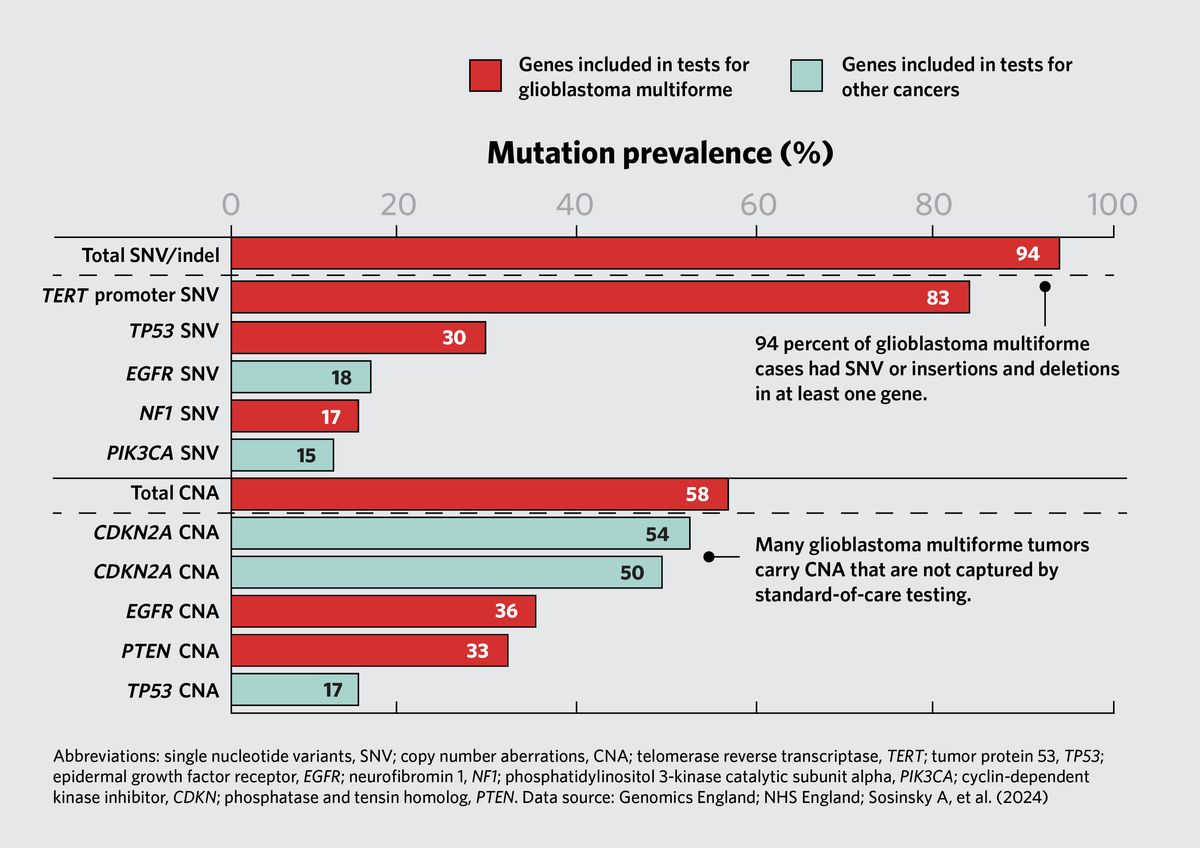
Caulfield and his team started by looking at the mutation rates for genes included in the most current version of the National Genomic Test Directory for Cancer (NGTDC), a list that specifies which genomic panels a patient is eligible for based on their specific cancer type. “The findings were quite striking,” said Caulfield. For example, they found that 94 percent of tumors from patients with glioblastoma multiforme, an aggressive form of brain cancer, had small variants in at least one gene, and 58 percent contained a CNA in at least one gene. Standard-of-care, targeted panels do not test for many of the observed mutations. “This complex genetic architecture led to the conclusion that to really characterize the genetic variation in glioma, a whole genome would be the best test,” said Caulfield.
The most frequently mutated gene across cancer types was tumor protein 53 (TP53). However, within individual cancer types, the occurrence of each mutation was variable, highlighted by a high frequency of TP53 SNV in ovarian high grade serous carcinoma (90 percent) and a low frequency of the mutation in mesothelioma (seven percent).
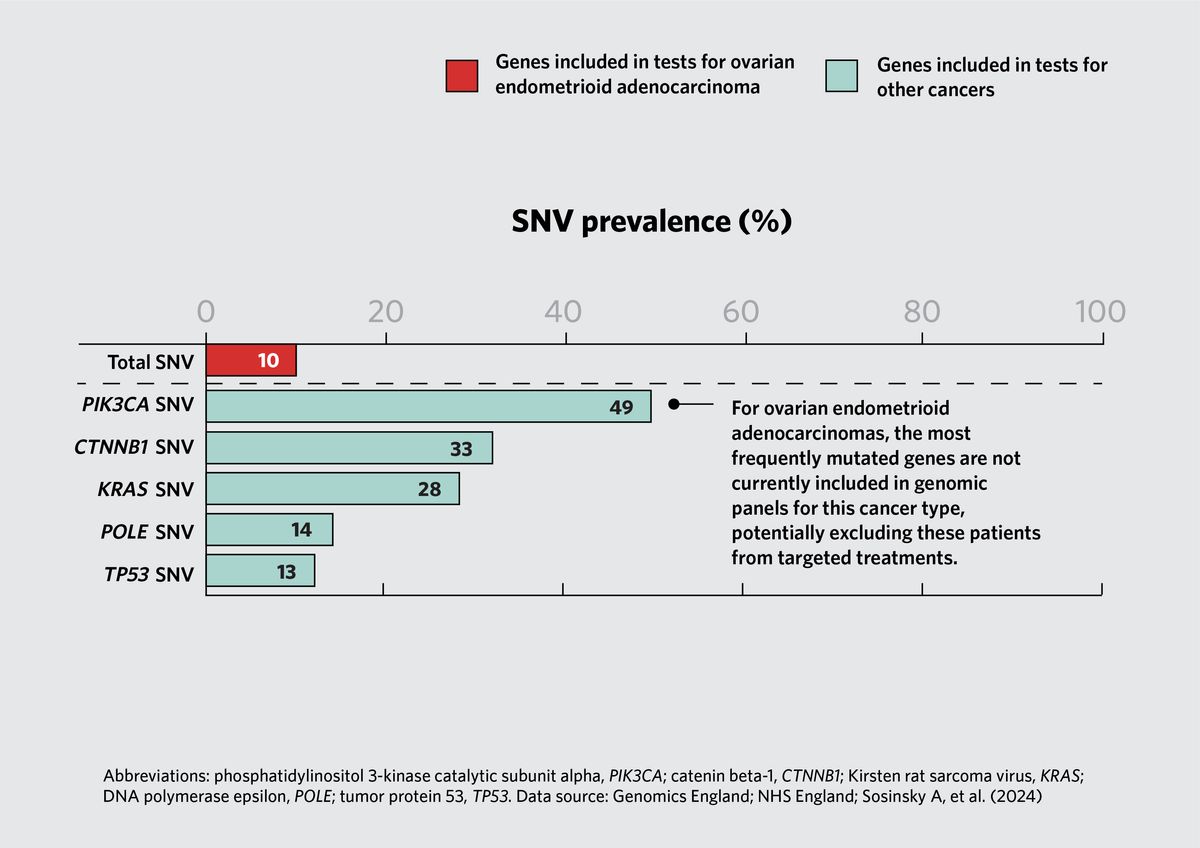
The research team also assessed mutation rates for genes that are not included in a cancer type’s standard-of-care testing protocol. For example, 54 percent of uterine corpus endometrial carcinomas and 49 percent of ovarian endometrioid adenocarcinomas carried a phosphatidylinositol 3-kinase catalytic subunit alpha (PIK3CA) mutation. However, in the UK healthcare system, this mutation is only tested in breast invasive carcinomas. These findings suggest that scientists could test the therapeutic potential of PIK3CA inhibitors in clinical trials for these cancers.
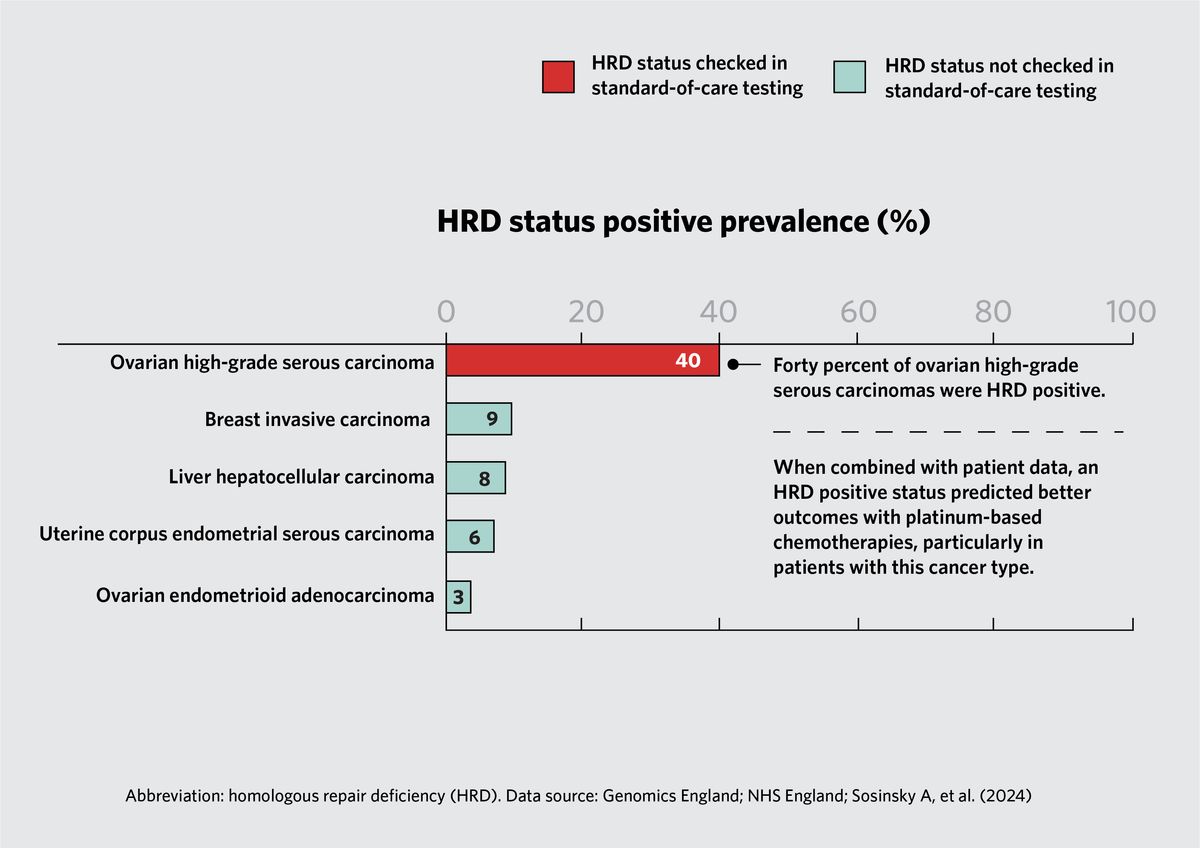
Homologous recombination deficiency (HRD) occurs when cells fail to repair double-stranded breaks in DNA via homologous recombination. Caulfield and his team found that 40 percent of ovarian high-grade serous carcinomas had genes with HRD. When they incorporated patient clinical data into their analysis, they found that patients whose tumors had high rates of HRD experienced better outcomes with platinum-based chemotherapies relative to patients with no genetic alterations in HR pathway genes; this was particularly true for patients with high-grade ovarian carcinomas.
Because the research team used an integrated WGS approach, they could sequence patient tumor and non-tumor DNA in tandem in search of variants that carry information about patient predisposition to malignancy that can guide their clinical management. For example, 13 percent of patients with high-grade serous ovarian carcinoma had variants in BRCA1 and BRCA2 genes. Patients with this cancer type had an earlier cancer onset if they carried predisposing germline variants. “If we could harness that information in families where there is a strong cancer history, it may be that we'd be able to do a better job of screening and focusing screening on specific individuals,” said Caulfield.
The current study only included WGS data, and thus the pipeline only accounted for a patient’s genomic profile. “It would have been appealing to have included RNA sequencing or transcriptome sequencing because that gives you an epigenetic framework for the tumor microenvironment,” said Chinnaiyan. “They're sort of missing the functional aspect of the genome that RNA sequencing brings.”
Regardless, he noted that the genomic findings were impressive and intriguing. “[The study] is going to have an impact in terms of the cancer research community,” said Chinnaiyan, whose research group is already getting access to the data to support ongoing studies.
When the 100,000 Genomes Project started in 2013, genetic testing in cancer was limited. WGS has revolutionized cancer research, unraveling the complex genetic architecture of cancerous cells; however, the technology is largely restricted to research.
“The future of cancer care will almost certainly involve some degree of either genomic, or possibly multi-omics profiling, which will allow us to more correctly choose the therapies for patients,” said Caulfield. Chinnaiyan similarly sees WGS as playing a major role in cancer care of the future. “The longer-term vision for precision oncology is to carry out this comprehensive approach if the costs can be managed,” said Chinnaiyan. Although the cost to sequence an entire human genome has dropped substantially since the days of the Human Genome Project, it is still prohibitively expensive once processing, analysis, interpretation, and storage costs get factored in.4,5
However, Caulfield hopes that day will come soon. He said, “Eventually, what will happen is that the price of a whole genome will fall down, and at that point, it's very likely that everyone will move towards whole-genome sequencing because it will be cost-effective. And what that will allow us to do is to detect unusual features in people's cancers that go beyond our current knowledge.”
Caulfield said that the next phase is to put WGS alongside clinical trials to understand whether they can identify biomarkers for treatment response. With the mutational architecture of a tumor and electronic clinical data in hand, researchers could follow a patient’s health journey beyond the end of a clinical trial.
- Sosinsky A, et al. Insights for precision oncology from the integration of genomic and clinical data of 13,880 tumors from the 100,000 Genomes Cancer Programme. Nat Med. 2024;30(1):279-289.
- Turnbull C, et al. The 100,000 Genomes Project: Bringing whole genome sequencing to the NHS. BMJ. 2018;361:k1687.
- Kurian AW, et al. Germline genetic testing after cancer diagnosis. JAMA. 2023;330(1):43-51.
- Akhoundova D, Rubin MA. The grand challenge of moving cancer whole-genome sequencing into the clinic. Nat Med. 2024;30(1):39-40.
- Schwarze K, et al. The complete costs of genome sequencing: A microcosting study in cancer and rare diseases from a single center in the United Kingdom. Genet Med. 2020;22(1):85-94.