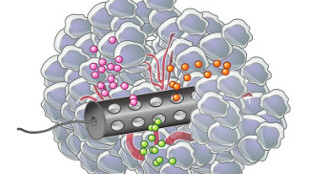
 MIT-developed device with three different drugs diffusing through tumorERIC SMITHDespite advances in analyzing tumor biology, choosing effective therapies for cancer patients remains difficult. This is partly because there are still no timely, foolproof ways to test whether a patient will respond to a particular treatment. Addressing this issue, two independent teams—one at the Fred Hutchinson Cancer Research Center in Seattle and another at MIT—have developed devices that can test a tumor’s response to multiple cancer drugs directly in the patient. Both devices are described today (April 22) in Science Translational Medicine.
MIT-developed device with three different drugs diffusing through tumorERIC SMITHDespite advances in analyzing tumor biology, choosing effective therapies for cancer patients remains difficult. This is partly because there are still no timely, foolproof ways to test whether a patient will respond to a particular treatment. Addressing this issue, two independent teams—one at the Fred Hutchinson Cancer Research Center in Seattle and another at MIT—have developed devices that can test a tumor’s response to multiple cancer drugs directly in the patient. Both devices are described today (April 22) in Science Translational Medicine.
If validated in human clinical studies, the devices—which are being further developed by spinoff companies—could be used before surgery to help identify the best course of individualized treatment for certain cancer patients.
“[These devices] are important because they capitalize on two aspects of the cancer problem. One is the fact that we have an enormous number of drugs that cannot possibly all be tested in all cancers using conventional trial methodologies,” R. Charles Coombes, a professor of medical oncology at Imperial College London who penned an accompanying editorial but was not involved in either study, wrote in an e-mail to The Scientist. The second, Coombes continued, is “the realization that all cancers differ from one another.”
“Rather than serially treating patients until the most effective ...