 Injection of an adenoviral vector encoding pancreatic transcription factors induced insulin expression (red) in mouse pancreatic ducts. Ductal tissue is shown in green and nuclei in blue. YUHAN WANGOne strategy to treat type 1 diabetes, where the immune system destroys insulin-producing β cells, is to convert other cells into β-like cells that then take over insulin production. In a study published today (May 2) in Molecular Therapy, researchers reprogrammed pancreatic duct cells in vivo to successfully treat both genetically and chemically induced diabetes in mice.
Injection of an adenoviral vector encoding pancreatic transcription factors induced insulin expression (red) in mouse pancreatic ducts. Ductal tissue is shown in green and nuclei in blue. YUHAN WANGOne strategy to treat type 1 diabetes, where the immune system destroys insulin-producing β cells, is to convert other cells into β-like cells that then take over insulin production. In a study published today (May 2) in Molecular Therapy, researchers reprogrammed pancreatic duct cells in vivo to successfully treat both genetically and chemically induced diabetes in mice.
“The work is part of a long line of studies attempting to genetically engineer insulin-producing cells in various tissues,” says Jake Kushner, a pediatric endocrinologist at Baylor College of Medicine in Houston who did not participate in the study. “It’s an exciting advance because it refines our understanding of what the capacity is of the various different components of either the pancreas or the liver to be transduced and make insulin-producing cells.”
Previous work had shown that both liver and pancreas cells could be converted into insulin-producing cells. Thus, Yuhan Wang, then a graduate student in Markus Grompe’s lab at Oregon Health & Science University, and colleagues set out to determine which of these cell types would make the best β-like cells.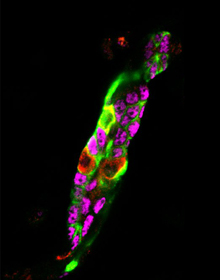 Injection of an adenoviral vector encoding pancreatic transcription factors induced insulin expression (red) in mouse pancreatic ducts. Lineage traced pancreatic ducts were identified by GFP (green). Insulin-positive pancreatic ducts were characterized by their loss of pancreatic ductal transcription factor expression, Sox9 (magenta).YUHAN WANG
Injection of an adenoviral vector encoding pancreatic transcription factors induced insulin expression (red) in mouse pancreatic ducts. Lineage traced pancreatic ducts were identified by GFP (green). Insulin-positive pancreatic ducts were characterized by their loss of pancreatic ductal transcription factor expression, Sox9 (magenta).YUHAN WANG
In the latest study, the researchers intravenously injected diabetic mice with a virus loaded with the genes for Pdx1, Neurog3, and Mafa—transcription factors that had already been shown ...



















