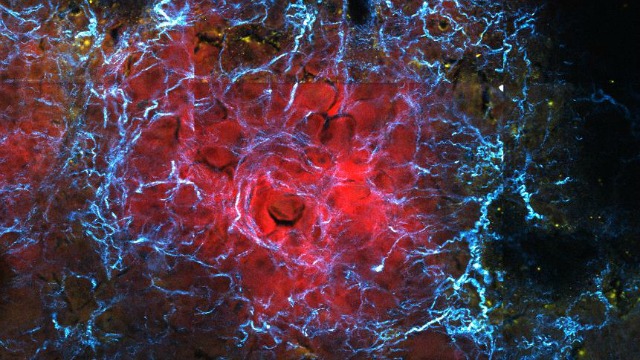
 The collagen network (cyan) serves as a biophysical biomarker of cancerous cells (red).LINAN LIU & JENU V. CHACKOFor the first time, researchers have genetically engineered mesenchymal stem cells (MSCs) to deliver a therapeutic to tumor cells based on their stiffness relative to surrounding tissue. In a study published today (July 26) in Science Translational Medicine, scientists at the University of California, Irvine, report harnessing MSCs’ ability to sense outside pressure to deliver anticancer medication to tumors in mice, reducing damage from the drug to non-cancerous tissue.
The collagen network (cyan) serves as a biophysical biomarker of cancerous cells (red).LINAN LIU & JENU V. CHACKOFor the first time, researchers have genetically engineered mesenchymal stem cells (MSCs) to deliver a therapeutic to tumor cells based on their stiffness relative to surrounding tissue. In a study published today (July 26) in Science Translational Medicine, scientists at the University of California, Irvine, report harnessing MSCs’ ability to sense outside pressure to deliver anticancer medication to tumors in mice, reducing damage from the drug to non-cancerous tissue.
“Stiffness as a biomarker . . . lasts for years in the body, so it’s very difficult for cancer to develop resistance if you target stiffness,” says senior author Weian Zhao.
Zhao says his group based the work on two recent observations: that biophysical cues play a role in cancer progression, and that stem cells can sense the stiffness of their environments and change their behavior accordingly.
Reasoning that stem cells’ stiffness-sensing properties could be used to deliver therapeutics in a targeted way, his team engineered human MSC genomes by placing a promoter, activated when the cells sense outside pressure, just upstream from a gene for the enzyme cytosine deaminase, which converts the anticancer drug 5-fluorocytosine to its active form.
Then the researchers injected the ...























